| Chapter 1: Structure Determines Properties |
| Chapter 1: Structure Determines Properties |
To test whether you know all that you need
to know you must try to answer
these questions before you look at the answers!
You may wish to refer to a periodic table
when answering the following questions.
1. How many electrons are there in N3-
?
2. What is the atomic mass unit for bromine ?
3. How many protons and neutons are there in each of the
following
: 1H, 2H, 12C, 16O, 35Cl
and 37Cl ?
1. Which quantum number defines the
energy level of an orbital
for (a) the hydrogen atom and (b) other atoms ?
2. What are the quantum numbers that describe a 3p orbital ?
3. How many occupied valence orbitals are there in Cl and I ?
4. What are the quantum numbers for an electron in a 3s orbital
?
Use the information given in the box below when answering the next three questions:
| (a) [Ar] | (d) 1s22s22p63s23px23py1 |
| (b) 1s22s22p63s1 | (e) 1s22s22p63s23px13py13pz1 |
| (c) 1s22s22p63p1 |
1. Which of the electron
configurations above best describes the
ground state electron configuration of Cl-?
2. Which of the electron configurations above is possible for
the ground state of P?
3. Which of the electron configurations above describes an
excited
state of Mg+?
4. Is the following electron configuration consistent with Hund's rule of maximum multiplicity?
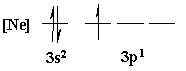
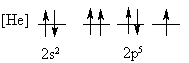
7. What is the orbital energy diagram that best describes the valence electron configuration of O?