| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
Bonding in "He2"
Why can't helium form bimolecular helium molecules (He2)
analogous to H2 ?
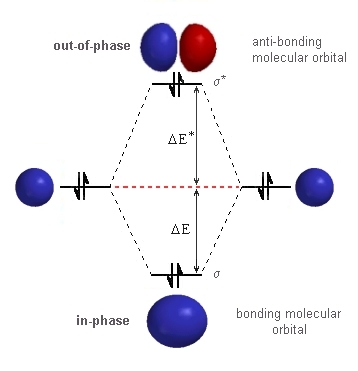
| We can use the same basic diagram that
we used for hydrogen since the same type of atomic orbitals, the 1s orbitals,
are involved. The only difference is that they now contain a pair of electrons.
Everything is the same until we put
in the electrons. The stabilisation due to the electrons in the σ-bonding orbital is given by 2 DE. The destabilisation due to the electrons in the σ* -anti-bonding orbital is give by 2 DE*. The critical factor from the quantum mechanical treatment is the magnitude of DE* > DE. Hence, the destabilisation due to the electrons in
the σ*-anti-bonding orbital is greater than the stabilisation
due to the electrons in the σ-bonding orbital. So a molecule of
He2 is less stable than 2 atoms of He ! |
 |
||
|
Conclusion
| © Dr. Ian Hunt, Department of Chemistry |