 |
Chapter 2 :
Alkanes |
 |
Thermodynamics and
Stability
- The lower the potential
energy of the system, the more stable it is.
- Chemical processes
usually occur because they are thermodynamically
favourable (i.e. DG = -ve)
- "Thermodynamically
favourable" means from high energy to low
energy,
or, put another way, from less stable to more stable.
- Understanding the
relative stability of molecules can be important for
predicting relative reactivity of starting materials and the relative
yields
of potential products.
- Stability can be
determined by comparing experimentally measured or
based
on theoretical calculations.
- It is important when
determining relative stabilty to compare isomeric
systems.
Basics
- DG is the change in the free energy of the system.
- DH is the change in the enthalpy of the system.
- DS is the change in the entropy (i.e. disorder) of the system.
- T is the absolute temperature in Kelvin.
- R is the universal gas constant, (R = 8.314 JK-1 mol-1 or 1.986 calK-1mol-1).
- K is the equilibrium constant.
There are two very important fundamental thermodynamic equations that you should know and understand that relate various thermodynamic terms:
DG = DH - TDS
DG = -RT ln K |
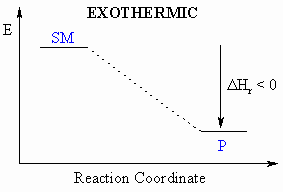
Heat of Reaction,
DHro
- Defined to be the heat
released during a particular reaction.
- If heat is released
during this process, then the
reaction
is exothermic ( = heat given out)
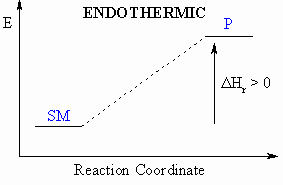
- If heat is absorbed
during this process, then the
reaction
is endothermic ( = heat taken in)
However, there are certain types of
reactions that are particularly
common for making thermodynamic comparisons:
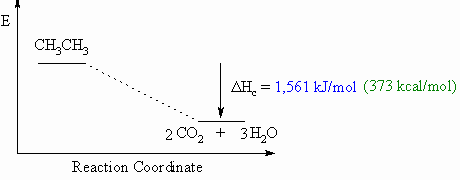
Heat of combustion, DHco
- Defined to be the heat
released when one mole of a compound undergoes
complete
combustion in O2.
- This will usually be an exothermic
process, as shown in the
example
below.
- When drawing these
diagrams, it is important to make sure they are balanced.
- Note that for heats of
combustion, the organic compound is a starting
material for the reaction.
 Heat of
formation, DHf
o
Heat of
formation, DHf
o
- Defined to be the heat
released if one mole of a compound were formed
from
its component elements in their standard state.
- These diagrams can be
either endothermic or exothermic
processes.
- When drawing these
diagrams, it is important to make sure they are balanced.
- Note that for heats of
formation, the organic compound is a product
for the reaction.
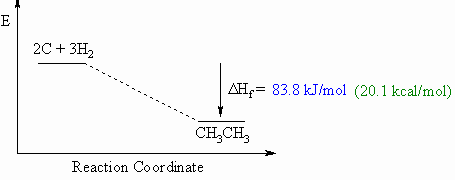 Heat of
hydrogenation, DHho
Heat of
hydrogenation, DHho
- Defined to be the heat released upon the addition of
H2 to one mole of a compound (e.g. an alkene or alkyne)
to generate the corresponding alkane.
- This will usually be an exothermic process, as
shown in the example below for ethene to ethane, since 2 σ bonds are made
from each π bond and each H-H σ bond.
- When drawing these diagrams, it is important to make
sure they are balanced (note the inclusion of both starting
materials).
- Note that for heats of hydrogenation, both starting
materials and products are organic compounds.
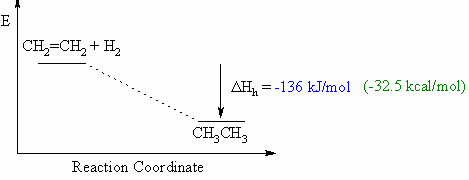
Hess's
Law
Although not specifically covered in most
organic text books, Hess's
Law is very useful when investigating the thermodynamics of
reactions.
Hess's Law can be expressed by the following expression and diagram:
"The enthalpy change for
a reaction (DHro)
that converts starting materials to products is independent of the
reaction
pathway"
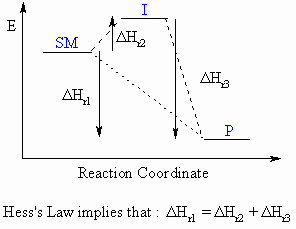 |
For the reaction SM to P which can either proceed
directly or via an intermediate, I, the overall enthalpy change for the
converssion of SM to P must be the same.
i.e.
DHSM-Po =
DHSM-Io + DHI-Po
|
Essentially, it can be treated as the addition of 2-dimensional
vectors, and CARE is required with the signs of the DHo
terms.