| Chapter 3: Conformations of Alkanes and Cycloalkanes |
| Chapter 3: Conformations of Alkanes and Cycloalkanes |
Alkanes
Let's start with simple alkanes so we can prepare ourselves for the more complex world that lies beyond.....
Methane: CH4|
Although σ bonds are able to rotate about the internuclear axis, the spherical symmetry of H atoms means that methane has a single unique conformation. |
||
| Rotation about the C-C bond in ethane (see the JMOL animation
to the right) produces different conformations. Although
an infinite number of conformations are possible, the staggeredeclipsed
conformation which represent the most and least stable respectively are
the two most important. Try rotating the 3D model of the animation so that you are looking directly along the C-C bond to see the interconversion of the two conformations. conformation and the |
||
| The differences between these two conformations are most apparent when viewed directly down the C-C bond, as in a Newman projection, see below: | ||
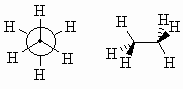
|
|
|
| Look at how the each H-C-H bond angle is bisected by a C-H bond on the adjacent C atom. This is the most stable conformation for ethane since the torsional strain is minimised. The staggered conformation is 12kJ/mol (2.9 kcal/mol) more stable than the eclipsed conformation (below). | ||
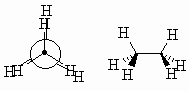 |
|
|
| Think like an astronomer....each of the C-H bonds is aligned with a C-H bond on the adjacent C atom so that the H attached to the front C obscures (eclipses) those on the rear C | ||
| Although there are 2 C-C bonds in propane, they are equivalent
and rotation produces conformations that are similar to those of ethane
except that the "extra" methyl group is interacting with the H atoms.
These can be seen by in the 3D model of propane
to the right. |
||
 |
© Dr. Ian Hunt, Department of Chemistry |