|
Structure and Preparation of Alkenes. Elimination Reactions |
|
Structure and Preparation of Alkenes. Elimination Reactions |
Elimination reactions
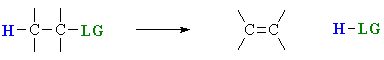
Elimination reactions are important as a method for the
preparation of alkenes.
The term "elimination" describes the fact that a small molecule
is lost during the process.
A 1,2-elimination or β-elimination indicates that the atoms that are lost come from adjacent
C atoms.
The two most important methods are:
Zaitsev's rule, based on the dehydration of alcohols, describes the preference for eliminations to give the highly substituted (more stable) alkene, which may also be described as the Zaitsev product. The rule is not always obeyed, some reactions give the anti-Zaitsev product.
Similarly, eliminations often favour the more stable trans-product
over the cis-product (stereoselectivity)
| © Dr. Ian Hunt, Department of Chemistry |