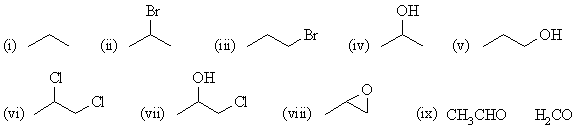
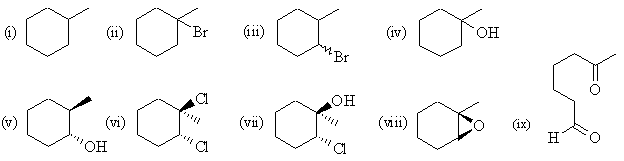
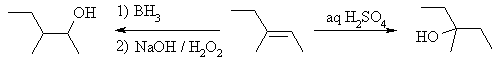| Qu 1: |
Pay attention to the regiochemistry and stereochemistry
in each case. |
|
(a) propene |
|
 |
|
|
|
(b) methylcyclohexene |
|
 |
|
|
| Qu 2: |
This question deals with the Markovnikov and anti-Markovnikov
hydration of alkenes. |
|
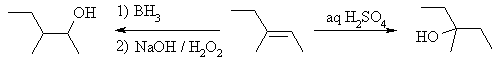
|
|
Aq. H2SO4
reacts by protonation of the alkene to yield the more stable 3o carbocation
followed by attack of H2O as the nucleophile and finally loss
of H+ to get the Markovnikov alcohol.
In contrast, borane (BH3) undergoes
a concerted addition, so there is no carbocation intermediate. The reaction
is controlled by a combination electronic and steric factors. The
B atom is the d+ and H d-
in this process (check the electronegativities of B and H). Thus, the B
adds at the least hindered end generating cation character at the more stable
carbocation site. This generates an alkyl borane. During the
oxidation step, an O atom is inserted into the C-B bond to give the C-O-B
system that generates the alcohol on reaction with NaOH. |
|
|
| Qu 3: |
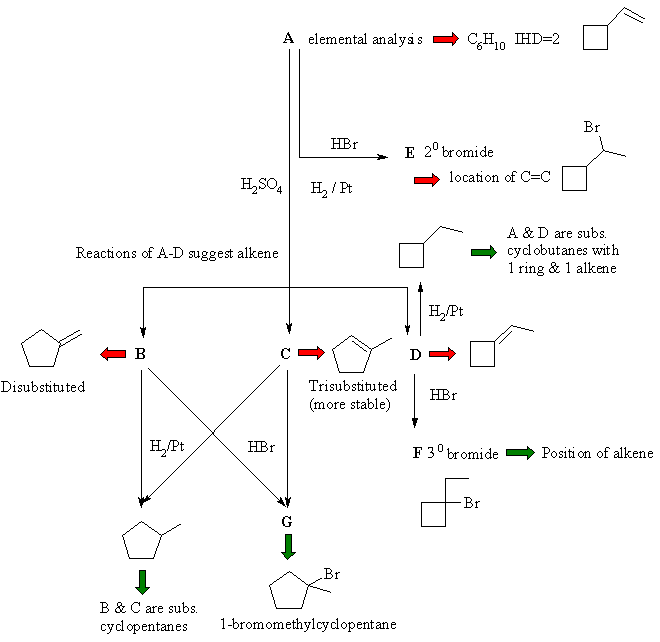
The following flow chart is constructed from the problem
and gradually built up to arrive at a solution. |
|
This type of problem is virtually impossible to slove
by "guessing" A then trying to work forwards from there. You need to extract
and sift through the chemical information to derive the solution.
Information in black comes straight from the problem, with deductions from
that indicated by red arrows. Green arrows indicate critical or key pieces
of information. In this question, the most likely route to solve it is probably
starting from G ...... |
|
|
|
|
|
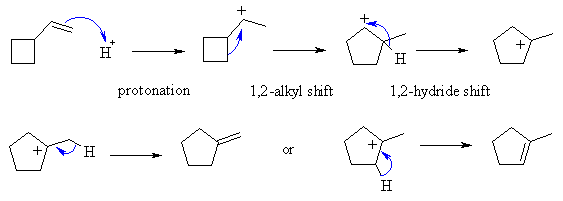
A reacts with the acid to form a 2o
carbocation, as we would expect is the first step of an acid addition reaction.
This carbocation can rearrange to give a more stable carbocation by an alkyl
shift that makes the 5 membered ring (less strain) which can give a 3o
carbocation via a hydride shift. Loss of a proton can then generate
two different alkenes.
|
|
|