| Chapter 7 : Stereochemistry |
| Chapter 7 : Stereochemistry |
So what structural features allow
a molecule to exist as a pair of enantiomers ? In order to be able to exist as a pair of enantiomers, a molecule requires the presence
of a chirality center - this is what we will be exploring here.
Terms such as an asymmetric, stereogenic or chiral center have
been used in the past but have now been replaced by the term chirality center in order to remove ambiguity.
Note that the term stereocenter is also used to describe a center that causes stereoisomerism. As such, an alkene can be a stereocenter.
In its simplest and most common case, a chirality center is characterised by an atom that has four different groups bonded to it in such a manner that it has a non-superimposable mirror image. The enantiomers of 2-chlorobutane we saw previously are shown below.
Questions :
Note you should be learning to relate the JSMOL images to the drawn images. More importantly you should be able to reproduce the drawn images since you may be expected to do this on examinations or assignments!
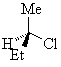
(R)-2-chlorobutane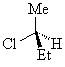
(S)-2-chlorobutane
The presence of a single chirality centre in a molecule results in a chiral molecule fundamentally because the top, bottom, left, right, front and back faces are all different.
Molecules can (of course) possess more than one chirality center. Such molecules may or may not be chiral.
Carbon-based Chirality Centers
The most prevalent chirality centers in organic chemistry are carbon atoms, which have four different groups bonded to them.
Chirality Centers other than Carbon
Any atom which has four different groups bonded to it is a chirality center. The more common of these atoms, with which an organic chemist should be familiar, are Si, N and P (note: a lone pair is included as one of the four different groups) They may be tetrahedral molecules or trigonal pyramidal molecules (where a lone pair is included as one of the four different groups)
CAUTION: many trigonal pyramidal molecules (especially those of nitrogen) exhibit rapid pyramidal inversion:
In this case, even though a chirality center is present in each molecule, the sample is optically inactive since the optical activity of the two extremes of inversion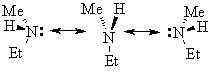
The larger the atom, though, the slower the pyramidal inversion and as a result many optically active compounds for P and S have been prepared.
| © Dr. Ian Hunt, Department of Chemistry |