| Chapter 7 : Stereochemistry |
| Chapter 7 : Stereochemistry |
The precise arrangement of substituents at a stereogenic center is known as the absolute configuration of the molecule.
If we have a drawn structure, then we can use the R- and S- notation system to assign the absolute configuration of that structure.
It is a more significant challenge to be able to assign the absolute configuration to an actual sample of a molecule (i.e. a compound in a bottle).
This is can be accomplished by solving the x-ray crystal structure of a molecule (a method that is not always readily available), by spectroscopic methods, or possibly by inference based on chemical
reactions of known specific stereochemistry involving a compound whose absolute configuration
is known.
Relative configuration can be applied within the same molecule (i.e. intramolecularly such as cis- or trans-) or it can be applied to intermolecularly (i.e. a pair of enantiomers have opposite configurations).
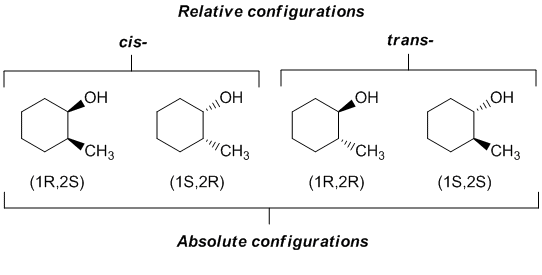
As an example, consider 2-methylcyclohexanol. There are two possible relative configurations based on the relative positions of the two substituents and whether they are on the same side or opposite faces of the cyclic structure. However, this system has two chirality centers and therefore there are 4 configurational isomers with the absolute configurations shown. This means that there are two possible configurations of the cis- structure.
The arrangement of atoms in an optically active molecule, based on chemical interconversion from or to a known compound, is a relative configuration. Relative, because there is no way of knowing just by looking at a structure whether the assignment of (+) or (-) is correlated to a particular enantiomer, R- or S-.
What does this mean ?
Suppose you have two samples X and Y that are pure enantiomers of each other, and you measure the rotation of each sample, and find let's say X = (+) and Y =(-).
Assuming you know the structure, you could then draw 3D-structures and assign the absolute configurations (R-) and (S-) to those drawn structures.
But how do you know which structure belongs to X and which one to Y ?
Answer... In general terms, based on this information alone you don't because there is no specific link or either R- or S- and (+) or (-). (R) could be (+) or (-). This is because it is not easy to relate the configuration of a drawn structure to the actual configuration of the molecules in the actual sample.
However, if we know for a specific case that (R) = (+), then for it's enantiomer, we do know that (S) = (-).
If the name of a compound includes both the sign of rotation and the designation R or S then the absolute configuration of that compound is known. This means other experiments have allowed the absolute configuration to the matched to a particular sample.
Let's see how chemists can determine
the relative configurations of optically active compounds by chemically interconverting
them.
The reaction of an alcohol with TsCl is known to occur with retention of configuration, that is the group priority of the stereogenic center has not been altered. The reaction of the tosylate with nitrile occurs with inversion, as a result the group priority at the stereogenic center has been altered. The absolute configuration of the parent is known while only the relative configurations of the tosylate and the nitrile are known. The mechanistic aspects behind this will be discussed in more detail in the next chapter.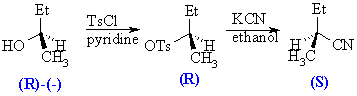
| © Dr. Ian Hunt, Department of Chemistry |