| Chapter 8: Nucleophilic Substitution |
| Chapter 8: Nucleophilic Substitution |
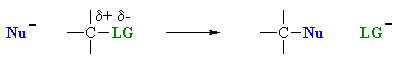
However, it is also useful to appreciate that the overall outcome of the transformation (i.e. the substitution) is often the same regardless of whether it is SN1 or SN2, though they may be differences in regiochemistry and / or stereochemistry (which can provide some evidence as to which mechanistic path is occuring).
IMPORTANT
In general terms, the C bearing
the LG needs to the sp3 hybridised
in order for these reactions to occur.
This is important since students often want to make use of nucleophilic substitution
reactions of vinyl (C=C-LG) or aryl (Ar-LG) systems which are not generally
effective.
The reasons for this are that the adjacent p bonds
are electron rich and will repel the Nu- and / or that the vinyl
and phenyl carbocations are not very favourable.
| © Dr. Ian Hunt, Department of Chemistry |