| Chapter 8: Nucleophilic Substitution |
| Chapter 8: Nucleophilic Substitution |
Nucleophilic Substitution Questions
| Qu 1: | Explain why cyclohexylamine (1) is more reactive than aniline (2) towards methyl iodide. |
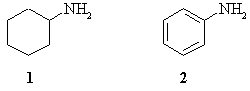
|
|
| Qu 2: | Explain the relative reactivity of the following alkyl bromides towards hydrolysis in aqueous ethanol solution: |
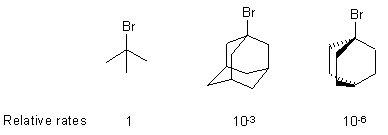
|
|
| Qu 3: | Rank the following in order of decreasing nucleophilicity. Explain your choice. |
|
|
|
| Qu 4: | Rank the groups (bold) in order of decreasing leaving group ability. Explain your choice. |
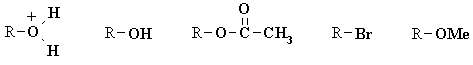
|
|
| Qu 5: | In a classic organic chemistry experiment,
the rate of the substitution reaction of radioactive iodide (isotope =128)
on (+)-(R)-2-iodooctane was investigated, see scheme below. The chemists
measured the initial rate of reaction for the incorporation of the iodine
isotope and also followed the progress of the reaction polarimetrically.
It was found that the initial rate of racemisation was twice the initial
rate of iodine incorporation. By considering the SN1 and SN2
in turn mechanisms, explain these experimental observations.
|
| Qu 6: | The base catalysed hydrolysis of the 1,2-chlorohydrin shown below is found to yield the 1,2-diol with retention of configuration. Suggest a mechanism that is consistent with this observation. |
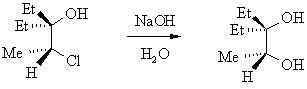
|
|
| Qu 7: | As part of a project in a petrochemical company, an achiral hydrocarbon, H, C4H8 was radically chlorinated. |
This gave a mixture of monochlorinated products, A, B, C, and D, that were then separated and characterised. It was found that both A and B existed as a pairs of enantiomers (i.e. A/A', B/B') and that A and B were diastereomers. The relative yields of the products was A = B > C > D (you could check this by calculating the % yields expected). In a series
of reactivity studies with AgNO3 / H2O / EtOH, the relative rates of reaction were C > A = B > D. Identify the monochlorinated isomers A, B, C and D and the original hydrocarbon H.
|
|
| Qu 8: | Draw a double headed curly arrow mechanism to account for the following experimental observation: |
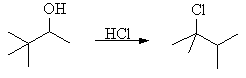
|
Answers ?
| © Dr. Ian Hunt, Department of Chemistry |