| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
Molecular Orbital Analysis of Ethene Dimerisation
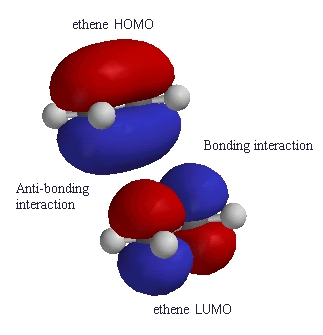
| Again compare the phases of the orbitals in the HOMO
of one ethene molecule with the phases of the LUMO of the ethene
molecule.
At one end there is a favourable bonding interaction (colours match) but there is an unfavourable anti-bonding interaction (colour mis-match) the other end. Therefore the reaction is said to be a "symmetry forbidden" - interestingly, this reaction is rare and very slow ! |
 |
A more complete analysis using molecular orbital methods (beyond our scope) can also explain:
| © Dr. Ian Hunt, Department of Chemistry |