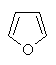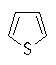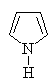 |
Chapter 11 : Arenes and Aromaticity |
 |
Heteroaromatics
Aromatic compounds which contain heteroatoms
(e.g. O, N, S) as part of the cyclic conjugated π system are called
heteroaromatics.
- The involvement of the heteroatom in the cyclic system requires that it
provides a p-orbital to be part of the conjugated π system.
- This is most common as either part of a π bond in the ring, or
a lone pair in a p-orbital to satisfy the criteria for aromaticity.
- This implies that the heteroatom be sp2 hybridised.
Study Tip:
Each heteroatom can only make ONE contribution to the π system.
This is because there can ONLY be ONE p orbital in the valence shell
with the appropriate geometry to interact with the other p orbitals
of the π system. There are three possible options:
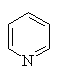
- The heteroatom is part of a π bond (e.g. pyridine)
- The heteroatom contributes a lone pair to the π system (e.g. furan)
- or it contributes an empty p orbital to the π system
Hence, in modes 1 and 2, the heteratom contributes 2 electrons to the π system
and in mode 3 it contributes none. |
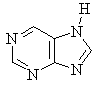
Several of the simpler and more common heteroaromatic
systems are shown below.
In each case consider role of heteroatom in the π system : is
it part of a π bond or does it contribute a lone pair to the
π system ?
Check the πsystem link to see.
* Purine is a precursor to the DNA bases Adenine and Guanine.