| Chapter 11 : Arenes and Aromaticity |
| Chapter 11 : Arenes and Aromaticity |
|
|
|
|
|
|
|
|
|
(phenyl bromide) |
|
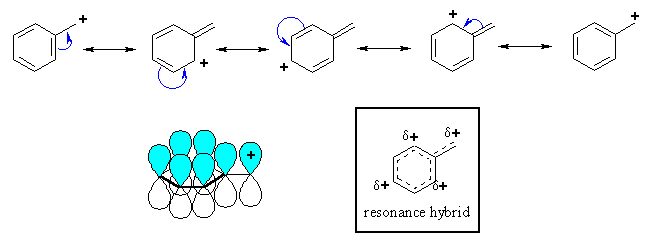 |
| The p system of a benzene ring can stabilise an adjacent carbocation by donating electron density through resonance. Remember that delocalising charge is a stabilising effect. |
Note that in the resonance forms
of the benzylic cation, the positive charge is located on the ortho and para
positions of the benzene ring, but not the meta positions. This is reflected
in the resonance hybrid.
Due to the stability of these benzylic cations, they are
readily formed as intermediates during chemical reactions, for example SN1
reactions of benzylic halides. Note that 2-chloro-2-phenylpropane is 600 times
more reactive that the 2-methyl analogue.
Benzylic radicals
Benzyl radicals can also be stabilised by resonance in the same manner as shown above for carbocations.
| © Dr. Ian Hunt, Department of Chemistry |