|
|
|
|
Why Substitution not Addition ?
As we just saw, electrophilic aromatic substitution can be represented as follows:
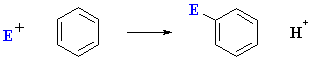
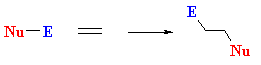
So the question is why don't arene C=C react in a similar fashion to alkene C=C and also
give overall addition ?
To answer this, we need to compare the two possibilities.
| The first step is the same in each of the pathways, E+ adds a C=C to give a carbocation intermediate. For an addition pathway, the nucleophile then adds to the electrophilic
carbocation giving a cyclohexadiene derivative. |
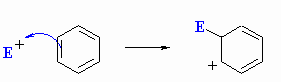
|
| For a substitution pathway, the "nucleophile" functions as a base and removes a proton from the sp3 C to recreate the C=C and restore the aromaticity. |
Recall that the resonance energy of benzene is about 152 kJ / mol (36 kcal / mol) and a conjugated diene is 16 kJ / mol (4 kcal/mol). This extra stability of the aromatic system is responsible for favouring the substitution reaction - substitution retains the aromatic unit whereas addition removes the C=C.
| © Dr. Ian Hunt, Department of Chemistry |