|
|
|
|
Sulfonation of Benzene

Reaction type: Electrophilic Aromatic Substitution
Summary
|
|
|
| Step 1: The p electrons of the aromatic C=C act as a nucleophile, attacking the electrophilic S, pushing charge out onto an electronegative O atom. This destroys the aromaticity giving the cyclohexadienyl cation intermediate. |
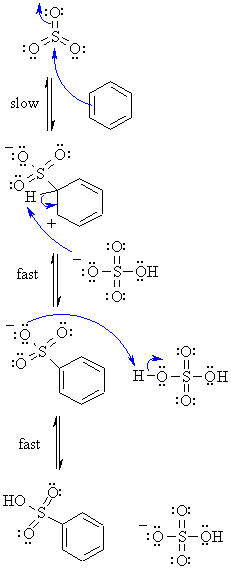
|
| Step 2: Loss of the proton from the sp3 C bearing the sulfonyl- group reforms the C=C and the aromatic system. |
|
| Step 3: Protonation of the conjugate base of the sulfonic acid by sulfuric acid produces the sulfonic acid.
|
|
| © Dr. Ian Hunt, Department of Chemistry |