 |
Chapter 12 :
Reactions of Arenes. Electrophilic Aromatic Substitution |
 |
Review of Limitations of Friedel-Crafts reactions
As we have seen previosuly there are two types
of Friedel-Crafts reactions, alkylation and acylation:
alkylation 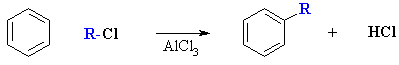
acylation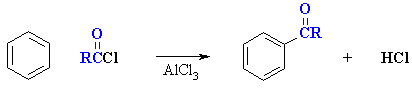
Reaction type: Electrophilic Aromatic Substitutions
However there are certain limitations:
Summary of Limitations of Friedel-Crafts alkylations:
- The halide must be either an alkyl halide.
Vinyl or aryl halides do not react (their intermediate carbocations
are too unstable).
- Alkylation reactions are prone to carbocation rearrangements.
- Deactivated benzenes are not reactive to Friedel-Crafts conditions, the
benzene needs to be as or more reactive than a mono-halobenzene (see substituent effects)
- Over alkylation can be a problem since the product is more reactive than
the starting material. This can usually be controlled with an excess of the benzene.
- The Lewis acid catalyst AlCl3 often complexes to aryl amines
making them very unreactive.
|
Summary of Limitations of Friedel-Crafts acylations:
- Acylation can only be used to give ketones. This is because HCOCl decomposes to CO and HCl under the reaction conditions.
- Deactivated benzenes are not reactive to Friedel-Crafts conditions, the
benzene needs to be as or more reactive than a mono-halobenzene (see substituent effects)
- The Lewis acid catalyst AlCl3 often complexes to aryl amines
making them very unreactive.
- Amines and alcohols can give competing N or O acylations rather than the require ring acylation.
|
QUESTION : Why aren't acylation reactions as prone to over
acylation ? ANSWER