|
|
|
|
Substituent Effects(contd.)
The following table shows the effect substituents have on both the rate and orientation of electrophilic aromatic
substitution reactions.
| Study Tip: This is a VERY important table ! It is worth knowing.... your best understanding will come if you learn HOW it works. It's application goes way beyond electrophilic aromatic substitution reactions. Key concepts to review ? Resonance and electronegativity |
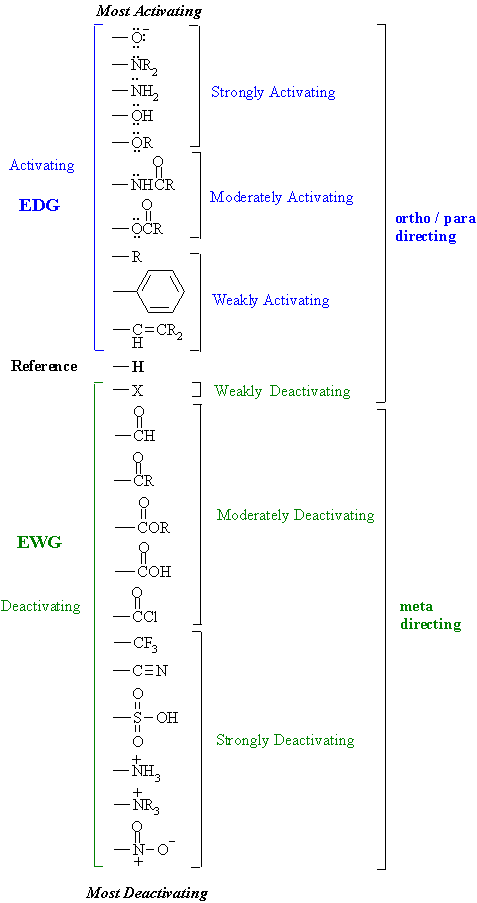 |
These effects are a combination of RESONANCE and INDUCTIVE
effects (see next page) The effects are also important in other reactions and properties (e.g. acidity of the substituted benzoic acids). Here are some general pointers for recognising the substituent effects:
|
| © Dr. Ian Hunt, Department of Chemistry |