 |
Chapter 12 :
Reactions of Arenes. Electrophilic Aromatic Substitution |
 |
Substituent Effects(contd.)
Study Tip:
Learn to recognise the types of substituents
and then break it down into the following :
(1) Electron donors activate and direct ortho and para.
(2) Electron withdrawing groups deactivate and direct meta.
(3) Except halogens.... they deactivate but direct ortho and para. |
First let's cover
the electronic effects :
1.
|
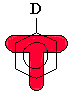
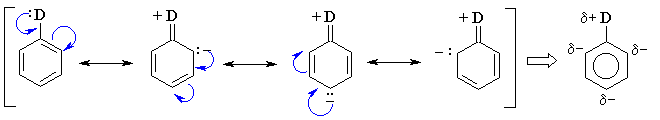
Substituents with lone pairs (e.g. -OCH3, -NH2)
on the atoms adjacent to the p system are electron donating
groups (EDG) - they activate the aromatic ring by increasing
the electron density on the ring through a resonance donating effect.
The resonance effect only allows electron density to be positioned at
the ortho- and para- positions. Hence these sites are
more nucleophilic, and the system tends to react with electrophiles
at these ortho- and para- sites. This is most easily
seen by pushing the curly arrows, see below :
|
 |
|
|
2.
|
Alkyl substituents (e.g. -CH3, -CH2CH3)
are also electron donating groups - they activate
the aromatic ring by increasing the electron density on the ring through
an inductive donating effect. This is the same effect that
allows alkyl groups to stabilise simple carbocations. They overall effect
is similar to that described above.
|
3.
|
Substituents with C=C (e.g. -vinyl or -aryl)
are also electron donating groups - they activate the aromatic ring by
a resonance donating effect. This is a similar effect to
that for type 1 except that the electrons are from a bonded pair not
a lone pair.
|
4.
|
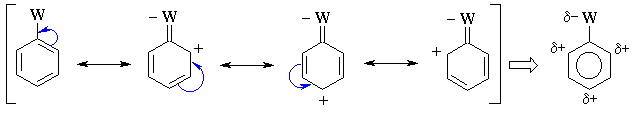
Substituents with pi bonds to electronegative atoms (e.g.
-C=O, -NO2) adjacent to the pi system are electron withdrawing
groups (EWG) - they deactivate the aromatic ring by decreasing
the electron density on the ring through a resonance withdrawing effect.
The resonance decreases the electron density at the ortho-
and para- positions. Hence these sites are less nucleophilic, and
so the system tends to react with electrophiles at the meta sites. |
 |
|
|
5.
|
Substituents with several bonds to electronegative atoms (e.g.
-CF3) adjacent to the pi system are electron withdrawing
groups (EWG) - they deactivate the aromatic ring by decreasing
the electron density on the ring through a inductive withdrawing effect.
The net overall effect is similar to that described above for other electron
withdrawing groups.
|
|
|
| 6. |
Halogen substituents are a little unusual
in that they are deactivating but still direct ortho- / para-.
The reason is that they are both inductive electron withdrawing (due to their
electronegativity) but they are also resonance
donating (lone pair donation). The inductive effect lowers
the reactivity of the starting material but the resonance effect controls
the regiochemistry due the stability of the intermediate carbocations. |
What about other
effects ?
Other than the electronic effects due
to resonance and induction described above, substitutents can also influence
product distributions in another way. For example consider the following experimental
data on the nitration of various alkyl benzenes :
| -R |
ortho-% |
meta-% |
para-% |
| -CH3 |
58
|
4
|
37
|
| -CH2CH3 |
45
|
6
|
49
|
| -CH(CH3)2 |
30
|
8
|
62
|
| -C(CH3)3 |
16
|
11
|
73 |
QUESTION
: Can you explain the trend in this data ? ANSWER
Other
thought provoking questions.....
- Why are esters (-OCOR) and amides (-NHCOR) less activating than ethers
(-OR) and amines (-NH)? ANSWER
- Why do esters and amides appear in the table twice, onceas an EDG
and once as an EWG? ANSWER
- Why are amines (-NH) better activators than alcohols (-OH)? ANSWER





