| Chapter 12 : Reactions of Arenes. Electrophilic Aromatic Substitution |
| Chapter 12 : Reactions of Arenes. Electrophilic Aromatic Substitution |
Electrophilic Aromatic Substitution Answers
| Qu 1 | |
(a) First a Friedel-Crafts alkylation reaction using EtCl to add an Et
group to the benzene followed by a radical bromination at the 2o
benzylic position (since it is the most stable radical), to give 1-bromo-1-phenylethane
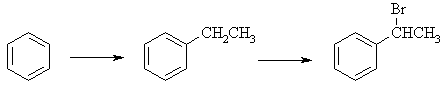
|
|
(b) Freidel-Crafts acylation of benzene will give the ketone without rearrangement
of the alkyl chain. The Wolff-Kishner reduction converts the C=O to a -CH2-
to give n-butylbenzene.
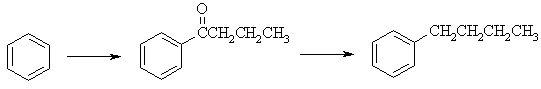
|
|
(c) FriedelCrafts alkylation of benzene with the 1o system,
isobutyl chloride will result in rearrangement via a 1,2-hydride shift to
provide the more stable 3o carbocation which alkylates the aromatic
to yield t-butylbenzene.
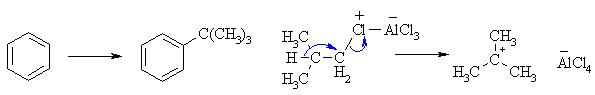
|
|
| Qu 2 | We are looking at a bromination reaction (but the same would be true of any of the other electrophilic aromatic substitution reactions) |
| This relative order of reactivity depends on the substituent on the benzene.
-Cl groups are weakly deactivating (induction), while -OH groups are strongly
electron donating (resonance with the lone pairs), and -NO2 groups
are strongly electron withdrawing (due to resonance onto the electronegative
O and induction due to the +ve N atom). Infact, phenols are reactive enough
to undergo polybromination even in the absence of the Lewis acid catalyst.
|
|
| Qu 3 | Since alkyl groups are weak electron donors and therefore slightly activating, t-butylbenzene will direct to the ortho and para positions. |
| However, the large size of the t-butyl group will sterically hinder both of the ortho positions and thus favour para substitution over ortho. Thus, iii > i > ii. | |
| Qu 4 | (a) This is a Friedel-Crafts acylation. The curly arrow mechanisms and charges may be drawn as follows |
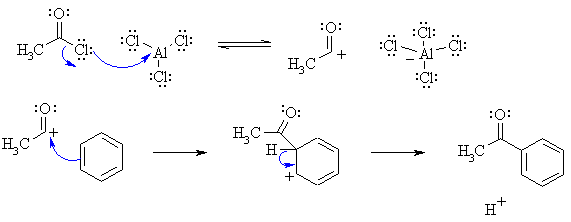
|
|
(b) This is a Friedel-Crafts alkylation. The curly arrow mechanisms
and charges may be drawn as follows:
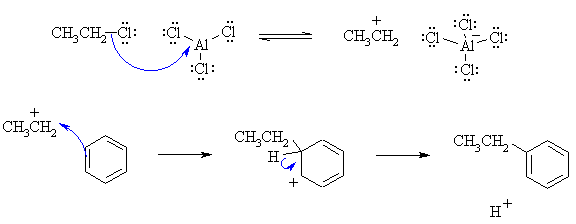
|
|
| Qu 5: | |
| We are looking at a Friedel-Crafts alkylation reaction (but the same would
be true of any of the other electrophilic aromatic substitution reactions).
This relative order of reactivity depends on the substituent on the benzene.
-CO2Me groups are deactivating (resonance withdrawal by the C=O),
while -OMe groups are strongly activating (resonance donation by the
-O- lone pairs), and -CH3 groups are weakly activating (inductive
donation due to polarisability and hyperconjugation).
|
|
| Qu 6: | Both starting materials are ester but the aromatic ring is connected to opposite sides of the carboxylate. |
| For ethyl benzoate, the aromatic ring has a -CO2CH2CH3
group attached which is an electron withdrawing group. Therefore the electrophilic
aromatic substitution reaction will occur at the meta position giving 3-nitrophenyl
ethanoate. For phenyl ethanoate, the aromatic ring has a -OC(=O)CH3 group attached. The -O- atom with it's lone pairs next to the ring makes this an electron donor, so ortho- and para- substitution occurs. Steric effects will favour the para- product. |
|
| Qu 7: | The resonance energy of naphthalene (61 kcal/mol) compared to benzene (36 kcal/mol) means that one of the rings is less aromatic than the other and will be more reactive. |
| © Dr. Ian Hunt, Department of Chemistry |