 |
Chapter 13: Spectroscopy |
 |
Index of Hydrogen Deficiency (IHD)
- The Index of Hydrogen Deficiency (IHD), is a count of how
many molecules of H2 need to be added to a structure in order to
obtain the corresponding saturated, acyclic species.
- Hence the IHD takes a count of how many multiple bonds and rings are present
in the structure.
- So, IHD can also be thought of as (multiple bonds + rings) or (π +
r).
- When you look at a structure, just count rings and π-bonds up (but
take care not to count any rings twice !)
- If you have a molecular formula, CcHhNnOoXx,
then the following equation (derivation shown below):
IHD = 0.5 * [2c+2-h-x+n]
Note that the IHD for neutral
organic molecules MUST be a positive integer (i.e. 0, 1, 2 etc.). If you calculate something different (e.g. 1.5 then there is an error!)
If you are trying to deduce the structure of an unknown molecule, and have the molecular formula, then the IHD should be calculated right away as it defines the type of structural features you need to consider.
There are two ways in which the IHD can be applied, and both can be useful depending up on the situation (a good student will be comfortable with both):
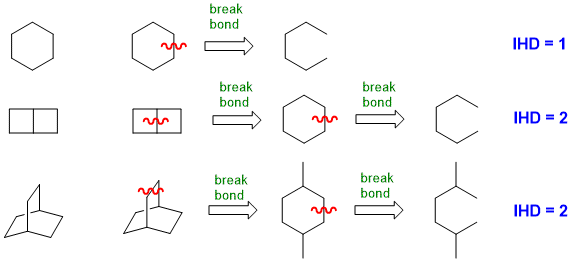
(1) From a drawn structure
When you look at a drawn structure, count
the number of π bonds and rings present (i.e. π + r) (but take care not
to count any rings twice !).
When counting π bonds, π bonds containing heteroatoms (e.g. O, N etc.) can be counted in exactly the same way as C π bonds.
- Each double bond has 1 π bond and therefore counts 1, each triple bond has 2 π bonds and counts 2
For simple ring systems this can be straight forward, but not as obvious for more complex ring systems.
- Count the number of bonds you need to "break" to make an acyclic (i.e. chain) structure:
(2) From the molecular formula
The IHD can be calculated directly from a molecular formula. Consider the following generic molecular formula CcHhNnOoXx,
then the following equation can be derived:
IHD = 0.5 * [ 2c + 2 - h - x + n ]
Where does this equation come from ?
- Well, the maximum
number of hydrogen atoms for "c"
carbon
atoms is 2c+2 (think of the formulae of saturated hydrocarbons such as
ethane, propane etc).
- From this maximum number,
subtract the "h" hydrogen atoms that you have.
- Since, like hydrogen, a
halogen atom only forms one bond, then they can be
treated
as if they are hydrogens, so subtract them as well.
- Oxygen forms two bonds,
therefore it has no impact (compare H
atom count
for methane, CH4, and methanol, CH3OH : both have 4 H).
- Nitrogen forms three
bonds. This means each nitrogen atom in a molecule, an extra
H atom is needed (compare the H count for methane, CH4, and
methyl amine, CH3NH2), therefore, for "n" nitrogen atoms present, add "n".
- The factor of 0.5
accounts for us counting H atoms, but
adding
hydrogen, H2 , molecules (because breaking one bond leaves two ends to be filled).
OK ?
Study Tip:
Make sure you can do it both ways!
With a more complex structure, counting C and H atoms to go from a drawing to a molecular formula risks making an error.
With a larger molecular formula, creating a drawing that you know fits the MF risks making an error.
Try the example below!
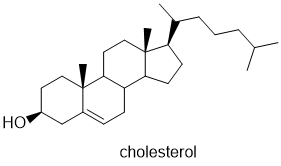 |
C27H46O |
ANSWER
|
Determining the IHD for molecules can be
useful for the following reasons:
- Seeing what types of
structural units are possible (e.g. an IHD of 1 means there needs to be a double bond or ring (but not both))
- Quickly checking
structures to see if they fit the molecular formula
rather
than simply counting H (when a mistake is possible)
QUESTIONS
- What is the IHD for each of the following molecular formulae ?
