| Chapter 13: Spectroscopy |
| Chapter 13: Spectroscopy |
Sample IR Spectra
:
By looking at IR spectra that contain known functional groups
and comparing and contrasting them with other IR spectra, one can develop the
skills required to be able to "interpret" an "unknown" IR spectra. Remember
that for an organic chemist, the primary role of IR is to identify the functional
groups that are present. A few examples reflecting some of the more important
functional groups are provided below.
Compare them to try to appreciate the subtle differences, comparing frequency,
intensity and shape.
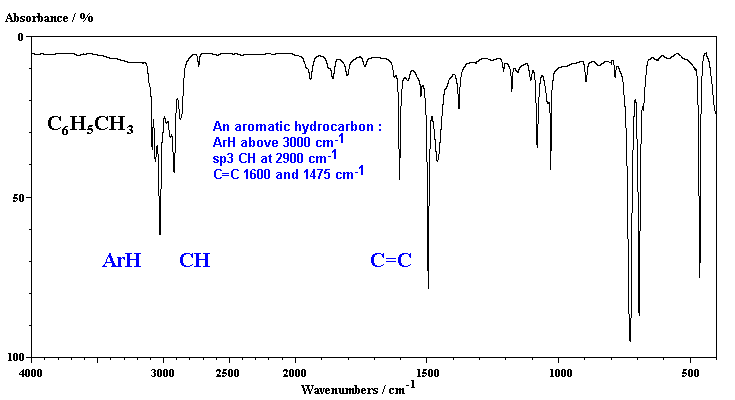
In the first example, of the aromatic hydrocarbon, toluene, we can see both the aromatic and aliphatic CH stretches, and two absorptions for the aromatic C=C stretches.
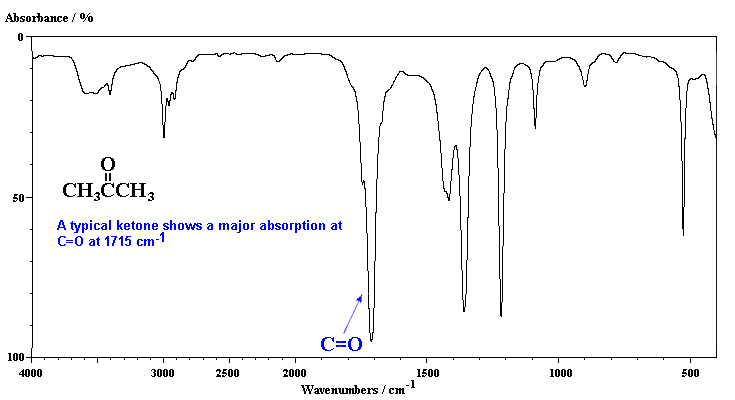
Acetone (2-propanone) is the "classic" carbonyl containing compound with the obvious C=O stretch in the middle of the spectra. Note that the peak is a very strong absorption. Compare it with the C=C in the previous case which are weaker and sharper.
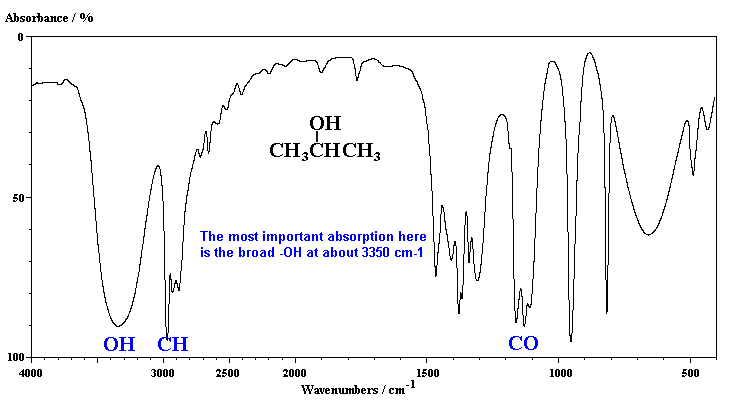
The characteristic absorption of the alcohol, 2-propanol, is the broad band due to the hydrogen bonded -OH group.
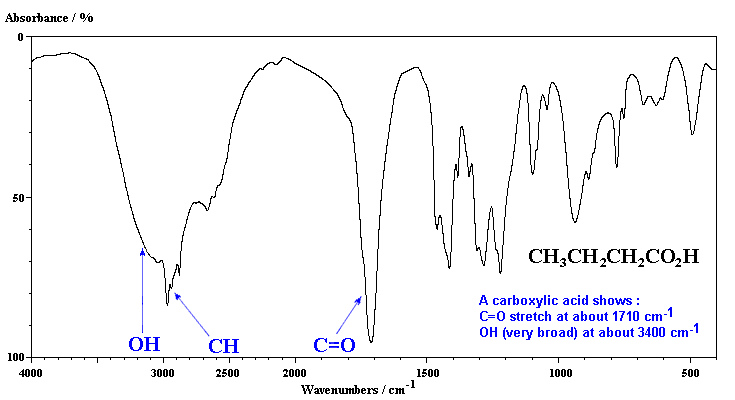
Carboxylic acids contain both C=O and OH groups. Note the broadness of both absorptions due to the hydrogen bonding and that the C=O is typically at slightly lower frequency than that of a ketone.
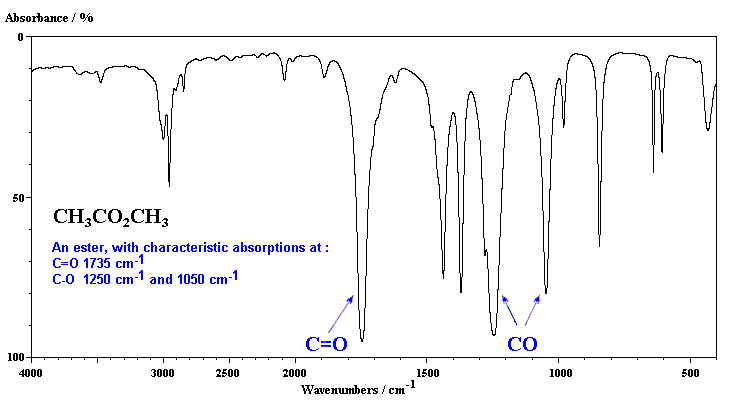
An ester has the follwoing key absorptions, the C=O and typically two bands for the C-O (not always easy to identify) since there are sp3 C-O and sp2 C-O bonds.
| © Dr. Ian Hunt, Department of Chemistry |