| Chapter 13: Spectroscopy |
| Chapter 13: Spectroscopy |
Molecular ion
IMPORTANT TIPS ! When calculating molecular weights to match to a molecular ion you need to use integer atomic weights. For low resolution MS, it is accurate enough to use H = 1, C = 12, N = 14, O = 16 etc. to check the MW of potential molecular formulae against the MW determined from the molecular ion in the MS. |
A mass spectrum is really just a frequency bar graph. The x axis is the mass to charge ratio (m / z), where z is usually 1 (i.e. +1 ions are formed). The y axis is the relative intensity (how often is the ion observed compared to the most common ion (the base peak)).
In the MS of cyclohexane, C6H12, shown below, we see a strong molecular ion at m/z = 84 (6 x 12 + 12 x 1 = 84), and many fragment ions including the base peak (the tallest peak) at m/z = 56. We are going to focus on the molecular ion:
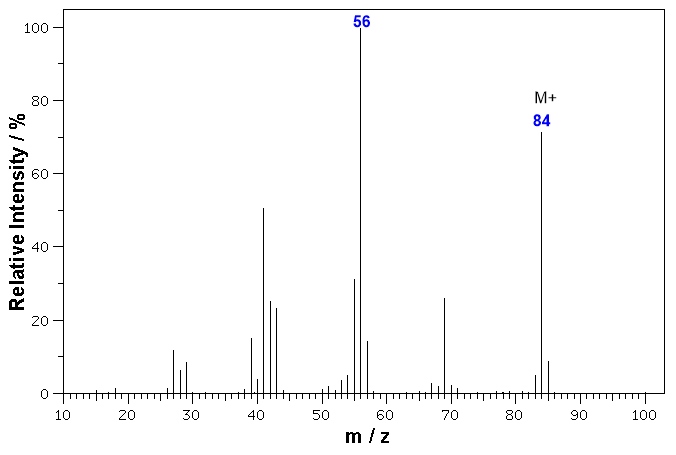
In this case we know the structure (cyclohexane, C6H12,) and hence the MW (84 g/mol). If we didn't know that, how would we identify the molecular ion ?
Remember that in a 100% pure sample, there shouldn't be anything heavier than the ion produced by the sample molecule.
So, the molecular ion (if present)* is going to be the heaviest ion in the MS (remembering to allow for the presence of isotopes). Remember that every peak has an "isotopic shadow".
Based on this, when we look at this MS, we see a group of ions at m/z = 83, 84, 85 and even a very tiny peak at 86.
The last "big" peak is the one at 84 which we assign as the molecular ion M+.
The peak at 85 is due to the small number of molecules that contain an atom of 13C (M+1), this 85 peak is the "isotopic shadow" of the peak at 84.
The peak at 86 is due to the even smaller number of molecules that contain two atoms of 13C (M+2) this 86 peak is the "isotopic shadow" of the peak at 85.
The peak at 83 must be due to loss of H from the molecular ion (M-1)
For smaller molecules, the M+ will always have a smaller M+1 peak an "isotopic shadow" due to the presence of 13C in molecules in the sample.
The more C atoms there are in a molecule, the larger the M+1 peak due to the presence of 13C will be.
Remember that for carbon, the majority is 12C but there will be a little 13C in the sample (which has about 1.1% natural abundance).
* if molecular ion is very weak or absent, then identifying the molecular ion of an unknown sample is much more difficult.
The nitrogen rule
Organic molecules composed of C, H, O and halogens have even molecular weights
Examples : CH4 = 16 g/mol, CH3CH3 = 30 g/mol, CH3OH = 32 g/mol, CH3F = 34 g/mol, CH3CO2H = 60 g/mol
Since N forms 3 bonds, compounds with an odd number of N atoms, have odd molecular weights
Examples : NH3 = 17 g/mol, CH3NH2 = 31 g/mol, C5H5N = 79 g/mol
Therefore, the implication is the nitrogen rule.... "an odd molecular weight implies the presence of an odd number of N atoms"
.
This can be a useful piece of information and prompt you to look for N containing functional groups in the IR spectrum.
| © Dr. Ian Hunt, Department of Chemistry |