| Chapter 13: Spectroscopy |
| Chapter 13: Spectroscopy |
Complex Coupling Patterns
In the following example, 3-bromopropene (also known as allyl bromide), we have coupling patterns that look more complex (frankly, kind of ugly!) compared to what we have seen before. Note that the C atoms are numbering in accord with the naming to help with the spectral analysis.
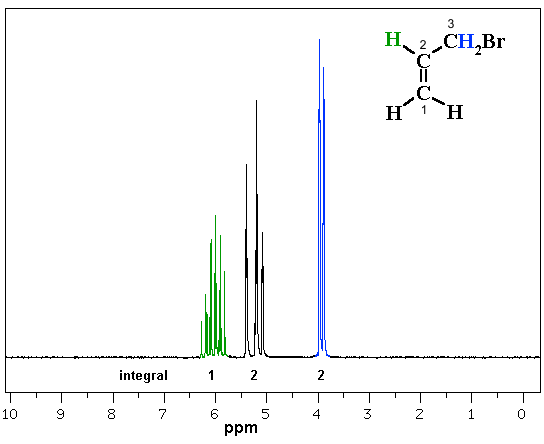 |
How many different types of H are there in 3-bromoprop-1-ene ? |
Let's talk it through...
The methylene H (2H, 4 ppm) on C3 looks like a doublet due to the coupling with the single H at C2. Note the chemical shift 4 ppm would agree based on proximity to -Br and the alkene.
The peaks at 5-5.5 ppm represents the 2 H (integration) at C1. The uneven spacing means it is not a triplet, it's more complex.... the 2H on C1 are not equivalent to each other which means that we are looking at two overlapping signals. Note the chemical shift of 5-6 ppm would agree with H-C=C.
The peaks at 5.8-6.3 ppm represent the H at C2. Clearly a complex pattern ! This H coupled to 3 different types of H each with different coupling constants which creates the complex pattern. Note the chemical shift of 5-6 ppm would agree with H-C=C.
| © Dr. Ian Hunt, Department of Chemistry |