| Chapter 13: Spectroscopy |
| Chapter 13: Spectroscopy |
Ultraviolet-Visible (uv-vis) Spectroscopy
Basics| Ultraviolet-visible spectropscopy (uv = 200-400 nm, visible = 400-800 nm) corresponds to electronic excitations between the energy levels that correspond to the molecular orbitals of the systems. In particular, transitions involving π orbitals and lone pairs (n = non-bonding) are important and so uv-vis spectroscopy is of most use for identifying conjugated systems which tend to have stronger absorptions. | |
| The lowest energy transition is that between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) in the ground state. The absorption of the EM radiation excites an electron to the LUMO and creates an excited state. The more highly conjugated the system, the smaller the HOMO-LUMO gap, DE, and therefore the lower the frequency and longer the wavelength, l. The colours we see in inks, dyes, flowers etc. are typically due to highly conjugated organic molecules. The unit of the molecule that is responsible for the absorption is called the chromophore, of which the most common are C=C (π to π*) and C=O (n to π*) systems. | 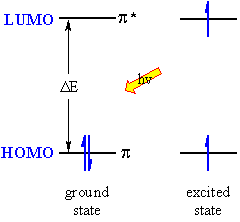 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Absorbance | A, a measure of the amount of radiation that is absorbed |
| Band | Term to describe a uv-vis absorption which are typically broad. |
| Chromophore | Structural unit responsible for the absorption. |
| Molar absorptivity | ε, absorbance of a sample of molar concentration in 1 cm cell. |
| Extinction coefficicent | An alternative term for the molar absorptivity. |
| Path length | l, the length of the sample cell in cm. |
| Beer-Lambert Law | A = ε.c.l (c = concentration in moles / litre) |
| λmax | The wavelength at maximum absorbance |
| εmax | The molar absorbance at λmax |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| © Dr. Ian Hunt, Department of Chemistry |