| Chapter 15: Alcohols, Diols and Thiols |
| Chapter 15: Alcohols, Diols and Thiols |
Oxymercuration-Demercuration
of Alkenes
(review of Chapter 14)

Reaction type: Electrophilic Addition
Summary
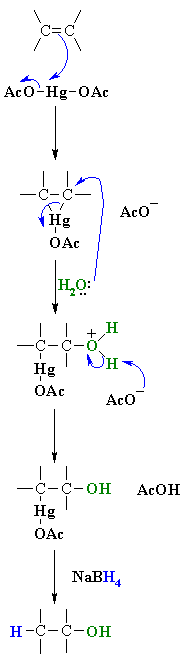
| MECHANISM
FOR REACTION OF ALKENES WITH Hg(OAc)2 / H2O
|
|
| Step 1:
The C=C p electrons act as the nucleophile with the electrophilic Hg and loss of an acetate ion as a leaving group, forming the mercurinium ion. |
|
| Step 2:
Water functions as a nucleophile and attacks one of the mercury substituted carbons resulting in cleavage of the C-Hg bond. |
|
| Step 3:
The acetate ion functions as a base deprotonating the oxonium ion to give the alcohol. This completes the oxymercuration part of the reaction. |
|
| Step 4:(mechanism
not shown) The hydride reduces the Hg off, creating a C-H bond while breaking the C-Hg bond. This is the demercuration part of the process. |
|
| © Dr. Ian Hunt, Department of Chemistry |