| Chapter 15: Alcohols, Diols and Thiols |
| Chapter 15: Alcohols, Diols and Thiols |
Reactions of RLi and
RMgX with Aldehydes and Ketones
(review of Chapter 14)
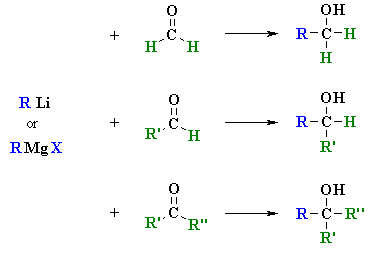
Summary
|
|
|
| Step 1:
The nucleophilic C in the organometallic reagent adds to the electrophilic C in the polar carbonyl group, electrons from the C=O move to the electronegative O creating an intermediate metal alkoxide complex. |
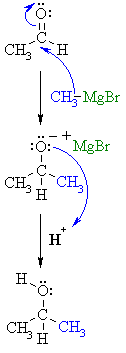
|
| Step 2:
This is the work-up step, a simple acid/base reaction. Protonation of the alkoxide oxygen creates the alcohol product from the intermediate complex. |
|
| © Dr. Ian Hunt, Department of Chemistry |