| Chapter 16: Ethers, Epoxides and Sulfides |
| Chapter 16: Ethers, Epoxides and Sulfides |
Ethers
Nomenclature:
Functional group suffix = ether (review)
Functional group prefix = alkoxy-
Ethers are said to be symmetrical if the two alkyl groups are the same, e.g.
diethyl ether.
|
methoxymethane |
ethoxyethane |
(MTBE) |
(THF) |
|
|
|
|
QUESTIONS
|
|
ANSWER | |
| ANSWER |
|
|
|
|
|
QUESTIONS
| ANSWER | ||
| ANSWER |
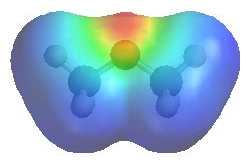 |
The image shows
the electrostatic potential for dimethyl ether. The more red an area is, the higher the electron density and the more blue an area is, the lower the electron density.
|
| © Dr. Ian Hunt, Department of Chemistry |