| Chapter 16: Ethers, Epoxides and Sulfides |
| Chapter 16: Ethers, Epoxides and Sulfides |
Using Halohydrins to
Synthesise Epoxides
(review of Chapter 6 and 8)
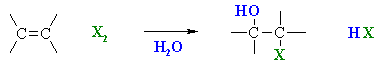
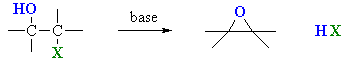
Summary
QUESTIONS
| ANSWER | ||
|
|
ANSWER | |
| ANSWER |
Related reactions
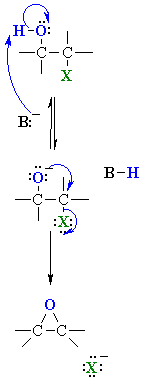
| MECHANISM
OF HALOHYDRIN TO EPOXIDE |
|
| Step 1:
An acid/base reaction. The base deprotonates the alcohol forming an alkoxide intermediate that has enhanced nucleophilicity. |
|
| Step 2:
An intramolecular SN2 reaction where the alkoxide nucleophile attacks the electrophilic C displacing the leaving group, the halide ion. The nucleophile has to attack anti to the C-X bond. |
|
| © Dr. Ian Hunt, Department of Chemistry |