| Chapter 17: Aldehydes and Ketones. Nucleophilic Addition to C=O |
| Chapter 17: Aldehydes and Ketones. Nucleophilic Addition to C=O |
Reactions of RLi and
RMgX with Aldehydes and Ketones
(review of Chapter 14)
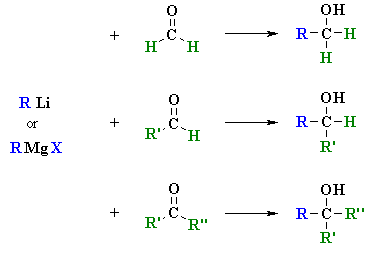
Reaction type: Nucleophilic Addition
Summary
NUCLEOPHILIC
ADDITION OF RLi or RMgX TO AN ALDEHYDE |
|
| Step 1: The nucleophilic C in the organometallic reagent adds to the electrophilic C in the polar carbonyl group, electrons from the C=O move to the electronegative O creating an intermediate metal alkoxide complex. |
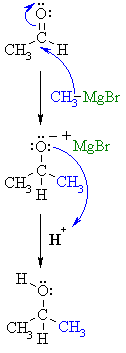 |
| Step 2: This is the work-up step, a simple acid / base reaction. Protonation of the alkoxide oxygen creates the alcohol product from the intermediate complex. |
|
| © Dr. Ian Hunt, Department of Chemistry |