|
|
|
|
Protecting Groups
 |
|
The overall transformation required is ester to primary alcohol. This is a reduction of the ester, which requires LiAlH4, but that will reduce the ketone as well which we don't want. We can avoid this problem if we "change" the ketone to a different functional group first. Conceptually, this is like being able to put a cover (shown below) over the ketone while we do the reduction, then remove the cover. |
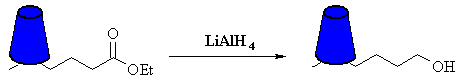 |
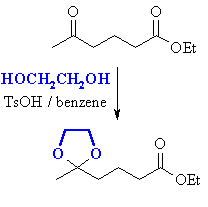
| In reality, the "molecular cover" is a protecting group. In this example, we protect the ketone as an acetal (which is an ether and doesn't react with LiAlH4) |
 |
|
Then we can reduce the ester to the primary alcohol. |
 |
|
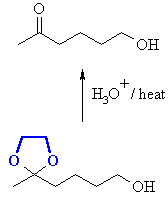
Finally we can remove the protecting group: |
 |
|
Overall, this gives us the complete scheme: 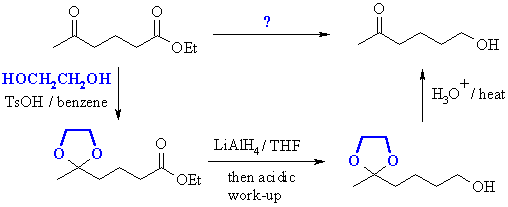 |
| © Dr. Ian Hunt, Department of Chemistry |