| Chapter 18: Enols and Enolates |
| Chapter 18: Enols and Enolates |
Compared to simple hydrocarbons,
the α-protons adjacent to carbonyl groups are much more acidic and can
be removed by common bases (e.g. HO-, RO- etc.).
For example, compare the acidity of propanone and propane :
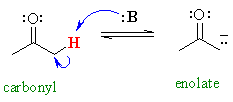 pKa = 19
pKa = 19 |
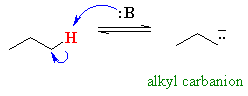 pKa » 50
pKa » 50 |
So the question is, why are the protons
adjacent to carbonyl groups acidic ?
As we have advocated before, we should
look at the stabilisation of the conjugate base.
Notice the proximity of the adjacent π system, and hence the possibility
for RESONANCE stabilisation by delocalisation of the negative charge
to the more electronegative oxygen atom.
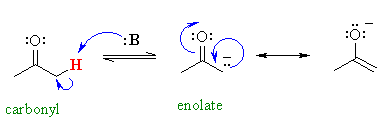
Can you think of any other simple examples similar to this situation ?
A very similar case is the comparison
of the acidity of carboxylic acids (pKa = 5) and alcohols (pKa
= 16). The acid is more acidic since the negative charge can be delocalised
to a second electronegative oxygen atom. This delocalisation makes the carboxylate
more stable (more favourable). This delocalisation is not possible in
the alcohol. Infact the carboxylic acid -OH proton is also an α-proton.
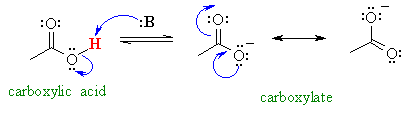 pKa = 5
pKa = 5 |
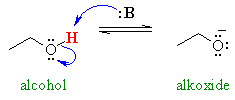 pKa = 16
pKa = 16 |
The same pattern occurs with the α-hydrogens in aldehydes and ketones... a resonance structure can be drawn with the negative charge relocated on the electronegative oxygen atom.....
The more effective the resonance stabilisation of the negative charge, the more stable the conjugate base is and therefore the more acidic the parent system.
Typical pKa values for a ketone and an aldehyde are shown:
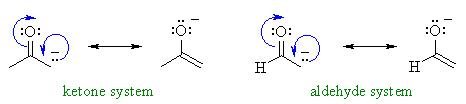 |
|
| pKa = 19 | pKa = 17 |
We can rationalise the trend by comparing
the two structures, the difference simply being the alkyl- group versus the
hydrogen.
Since alkyl groups are weakly electron donating, they tend to destabilise
anions (you should recall that they stabilise
carbocations).
This is because they will be "pushing" electrons towards a negative system which
is unfavourable electrostatically.
Hence the anions of ketone where there are extra alkyl groups are less
stable than those of aldehyde, and so, ketones are less acidic.
In some cases there could be H atoms that are adjacent to 2 carbonyl groups. This means that there is more resonance stabilisation of the anion since the charge can be delocalised to 2 electronegative oxygen atoms. As a result, we have an even more acidic pKa. These type of compounds are sometimes called "active methylenes".
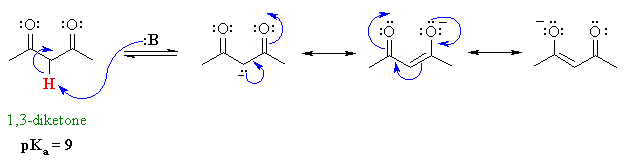
QUESTION A different system, but related type of scenario, is the hydrocarbon 1,3-cyclopentadiene which has a pKa = 16. Explain ANSWER
| © Dr. Ian Hunt, Department of Chemistry |