| Chapter 18: Enols and Enolates |
| Chapter 18: Enols and Enolates |
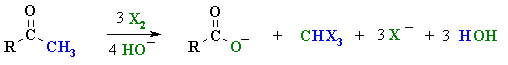
Summary
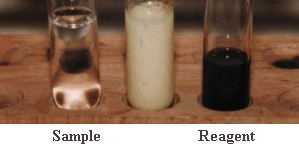
QUESTION Could a methyl aldehyde undergo this reaction ? ANSWER
Related Reactions
| MECHANISM OF THE HALOFORM REACTION OF METHYL KETONES | |
| Step 1: First, an acid-base reaction. Hydroxide functions as a base and removes the acidic α-hydrogen giving the enolate. |
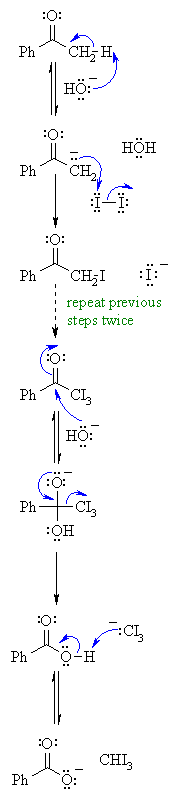 |
| Step 2: The nucleophilic enolate reacts with the iodine giving the halogenated ketone and an iodide ion. |
|
| Step 3: Steps 1 and 2 repeat twice more yielding the trihalogenated ketone. |
|
| Step 4: The hydroxide now reacts as a nucleophile at the electrophilic carbonyl carbon, with the C=O becoming a C-O single bond and the oxygen is now anionic. |
|
| Step 5: Reform the favourable C=O and displace a leaving group, the trihalomethyl system which is stabilised by the 3 halogens. This gives the carboxylic acid. |
|
| Step 6: An acid-base reaction. The trihalomethyl anion is protonated by the carboxylic acid, giving the carboxylate and the haloform (trihalomethane). |
|
| © Dr. Ian Hunt, Department of Chemistry |