| Chapter 19: Carboxylic Acids |
| Chapter 19: Carboxylic Acids |
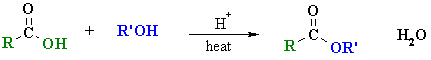
Summary
| Study Tip: The carboxylic acid and alcohol combination used to prepare an ester are reflected by the name of the ester, e.g. ethyl acetate (or ethyl ethanoate), CH3CO2CH2CH3 can be made from CH3CO2H, acetic acid (or ethanoic acid) and HOCH2CH3 (ethanol). This general "disconnection" is shown below: 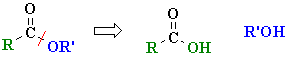
|
|
|
|
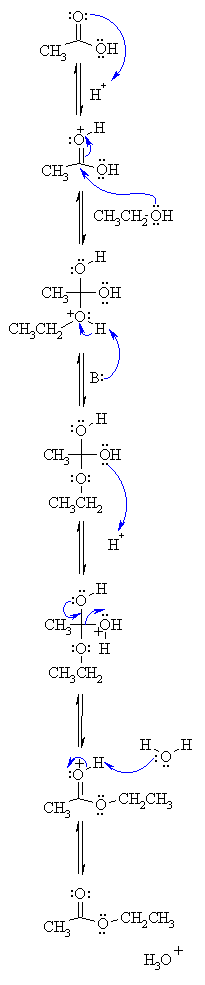
| Step 1:
An acid/base reaction. Protonation of the carbonyl makes it more electrophilic. |
|
| Step 2:
The alcohol O functions as the nucleophile attacking the electrophilic C in the C=O, with the electrons moving towards the oxonium ion, creating the tetrahedral intermediate. |
|
| Step 3:
An acid/base reaction. Deprotonate the alcoholic oxygen. |
|
| Step 4:
An acid/base reaction. Need to make an -OH leave, it doesn't matter which one, so convert it into a good leaving group by protonation. |
|
| Step 5:
Use the electrons of an adjacent oxygen to help "push out" the leaving group, a neutral water molecule. |
|
| Step 6:
An acid/base reaction. Deprotonation of the oxonium ion reveals the carbonyl in the ester product. |
|
| What is the B: used here ? If we look at the reaction, there are several bases present already. It could be the conjugate base A- of the acid catalyst, or a C=O group (as in step 1), or an R-OH group as in step 4, or a water molecule as in step 6. | |
| © Dr. Ian Hunt, Department of Chemistry |