| Chapter 25 : Carbohydrates |
| Chapter 25 : Carbohydrates |
The D- and L- notation is based on these structures. (Note that D- and L- means something quite different to d- and l-).
S-(-)-glyceraldehyde
or
L-glyceraldehydeR-(+)-glyceraldehyde
or
D-glyceraldehyde
| Study Tip ?
L-glyceraldehyde has the -OH group on the left
and
D-glyceraldehyde on the right in the Fischer projections of the
structures.
|
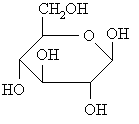
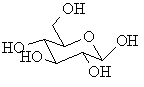
A series of carbohydrates are shown below, decide whether they are D- or L- by comparing them to glyceraldehyde
Haworth diagrams these are "flattened" diagrams used to represent the stereochemistry carbohydrates, note how the relative positions of the groups in the two structures are related.
L-glyceraldehyde ANSWER
 |
 |
|
|
| © Dr. Ian Hunt, Department of Chemistry |