| Chapter 26: Lipids |
| Chapter 26: Lipids |
Lipid Answers
| Qu 1: | (a) An unsaturated triglyceride must contain alkene C=C and contains 3 ester groups, RCO2R | ||||||
| (b) See the basic mechanism for a simple ester here. | |||||||
| Qu 2: | (a) Increasing the chain length will increase the Van der Waals forces and so increase the melting points. | ||||||
| So C18 > C16 > C12 or stearic (70oC) > palmitic
(63oC) > lauric (44oC)
(b) Increasing the degree of unsaturation lowers the melting point because
it interferes with the packing of the hydrocarbon chains.
|
|||||||
| Qu 3: | A possible set of isoprene units is shown for each structure below (there may of course be other possible answers) | ||||||
|
|||||||
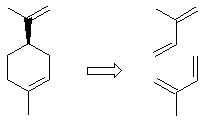
| Qu 4: | A Diels-Alder reaction (the hint should have been recognising the cyclohexene unit): | ||||||
 |
|||||||
| Qu 5: | The oil or grease particles becomes solubilised by becoming trapped in the hydrocarbon core of a micelle. | ||||||
| They are then carried into the aqueous solution and is washed away.
This is shown schematically below, the black circles represent grease particles.
|