| Qu 1: |
Why are the non-polar side chains unreactive ? |
|
|
| Qu 2: |
Does L-cysteine have R- or S- absolute configuration ? |
|
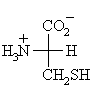 L-cysteine
L-cysteine
|
|
|
| Qu 3: |
Regular carboxylic acids such as ethanoic acid, have pKas around 5,
while in α-amino acids, the carboxyl pKa =2.
Why is this ? |
|
|
| Qu 4: |
The amino acid histidine has two potentially basic N sites in the side
chain, which one is more basic and why ? |
|
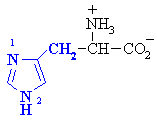
|
|
|
| Qu 5: |
What is the isoelectronic point, pI, of each of the following hypothetical
amino acids for which you are given their pKa values ? |
|
(pKa1 = carboxyl, pKa2 = ammonium, pKa3
= side chain functional group)
(a) pKa1 = 2.0, pKa2 = 10.0
(b) pKa1 = 2.0, pKa2 = 10.0, pKa3
= 4.0
(c) pKa1 = 2.0, pKa2 = 10.0, pKa3
= 8.0 |
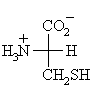 L-cysteine
L-cysteine 