| Useful Concepts |
| Useful Concepts |
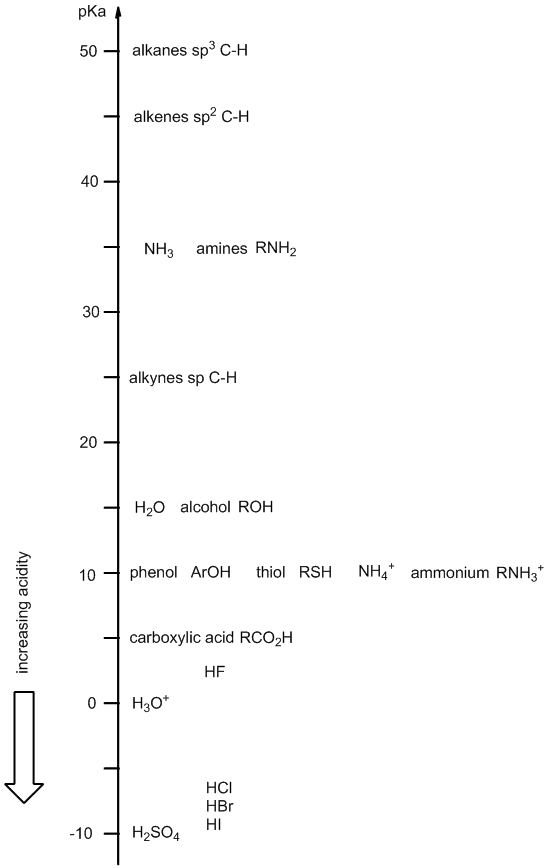
Acidity ladder
Derivation .... The "acidity ladder" gives a visual representation of the relative acidity of common organic functional groups and other common acids.
It provides an alternate method for recalling pKas or relative acidity / basicity order rather than memorising the numbers in a table.
The position on the ladder can be helpful in rationalising or predicting the reactions of acids and bases. We will talk about how to use the ladder after introducing it.
Rather than memorising absolute values, having a good knowledge of the general order of pKas can often be good enough, more than enough in fact..... and fortunately, the most important pKa values can often be rounded off to the nearest "5".... (the rungs of the ladder).
The acidity ladder shows the approximate pKa of the corresponding acid, for example, the pKa of water is about 15 (actual value = 14) corresponding to the reaction H2O <=> H+ + HO-.
When using the "ladder" make sure that you are considering the correct acid (especially for systems like water (alcohols) and amines) since they have two equilibria and both appear on the ladder. This is best done by making sure you write out the appropriate acid / base reaction and then identify the acid involved.
There are (of course) other organic functional groups that could be included to the acidity ladder. Feel free to print it out and add more values if it will help you. The functional groups represented on this version of the acidity ladder are the more common ones and help reveal some of the key general trends based on structural factors.
In this diagram, the ladder shows the acid species (HA) aligned with its corresponding pKa (on the next page is a smaller set of structures but the conjugate bases are also included).
Due to the nature of the pKa scale, a lower pKa corresponds to a stronger acid so the more acidic a species is, the lower it is on the ladder, e.g. sulfuric acid, H2SO4 (a strong acid) appears at the bottom of the ladder.
| © Dr. Ian Hunt, Department of Chemistry |