| Useful Concepts |
| Useful Concepts |
The Index of Hydrogen Deficiency (or IHD) is also known as "units of unsaturation" and several other similar names.
The index of hydrogen deficiency is
a count of
how many molecules of H2 need to be added to a structure
in order to obtain the corresponding saturated, acyclic species. Hence, the IHD takes a count of how many rings and multiple bonds are present
in the structure, so the IHD can
also be thought of as (multiple bonds +
rings)
or
Note that the IHD for neutral organic molecules MUST be a positive integer (i.e. 0, 1, 2 etc.). If you calculate something different (e.g. 1.5 then there is an error!)
There are two ways in which the IHD can be applied, and both can be useful depending up on the situation (a good student will be comfortable with both):
(1) From a drawn structure
When you look at a drawn structure, count the number of π bonds and rings present (i.e. π + r) (but take care not to count any rings twice !).
When counting π bonds, π bonds containing heteroatoms (e.g. O, N etc.) can be counted in exactly the same way as C π bonds.
For simple ring systems this can be straight forward, but not as obvious for more complex ring systems.
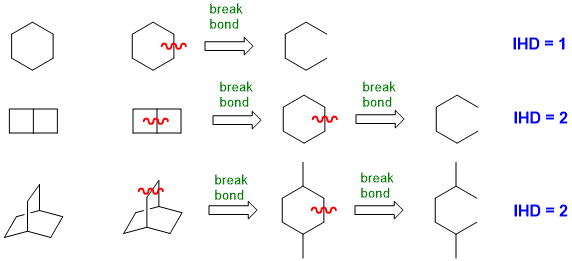
(2) From the molecular formula
The IHD can be calculated directly from a molecular formula. Consider the following generic molecular formula CcHhNnOoXx, then the following equation can be derived:
IHD = 0.5 * [2c+2-h-x+n]
Where does this equation come from ?
Study Tip: Try the example below !
|
Determining the IHD for molecules can be useful for the following reasons:
| © Dr. Ian Hunt, Department of Chemistry |