| Useful Concepts |
| Useful Concepts |
A leaving group , LG, is an atom (or a group of atoms) that is displaced as stable species taking with it the bonding electrons. Typically the leaving group is an anion (e.g. Cl-) or a neutral molecule (e.g. H2O). The better the leaving group, the more likely it is to depart.
A "good" leaving group can be recognised as being the conjugate base of a strong acid.
What do we mean by this ? First we should
write the chemical equations
for the two processes:
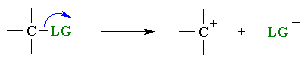 |
These two equations represent Bronsted
acid dissociation and loss of
a leaving group in a SN1 type reaction.
Note the similarity of the two equations: both show heterolytic
cleavage
of a s bond to create an anion and a cation.
For acidity, the more stable A- is, then the more the equilibrium will favour dissociation, and release of protons meaning that HA is more acidic.
For the leaving group, the more stable LG- is, the more it favours "leaving".
Hence factors that stabilise A- also apply to the stablisation of a LG-.
Here is a table classifying some common
leaving groups that we will
eventually meet......
| Excellent | TsO-, NH3 |
| Very Good | I-, H2O |
| Good | Br- |
| Fair | Cl- |
| Poor | F- |
| Very Poor | HO-, NH2-, RO- |
Note, once again, that HO- is a
poor leaving group (remember
from the reactions of alcohols ?).... after all its the conjugate base
of water.... and when we turn on a tap in the kitchen, we aren't
usually
trying to get a strong acid to drink !
But water itself, H2O, is a good leaving group, since it
is the conjugate base of H3O+, which is a strong
acid.
| © Dr. Ian Hunt, Department of Chemistry |