| Useful Concepts |
| Useful Concepts |
Nucleophilicity versus Basicity
Nucleophilicity and basicity are very similar properties in that species that are nucleophiles are usually also bases (e.g. HO- , RO-).
This is not too surprising since in the LEWIS sense, they are functioning as ELECTRON PAIR donors (i.e. both are LEWIS BASES), compare the two pairs of reactions mechanisms shown below to convince yourself.
However, it can avoid confusion by keeping these two types of reactivity separated because there are important differences....
1. Nucleophilicity:
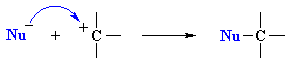
|
Both these reactions depict a nucleophile reacting with an electrophilic C atom |
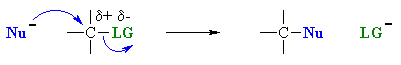 |
Kinetically controlled reactions of electron pair donor with an electrophilic carbon (usually) atom resulting in the formation a new C-X bond.
| Both these reactions depict a base reacting with an electrophilic H atom, a proton | |
Equilibrium (thermodynamic) reaction of the electron pair donor with a proton, forming a new H-X bond
The following general reaction mechanisms
show you why it is important
to appreciate the differences, since an anion that is reacting as a
nucleophile
will result in a substitution, but if it reacts as a base, then
elimination
will result, be it on a carbocation (SN1 vs E1) or with the
loss of a leaving
group (SN2 vs E2).
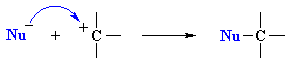
|
SN1 | |
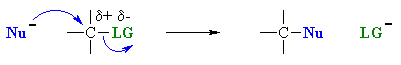
|
SN2 | |
| Eliminations | 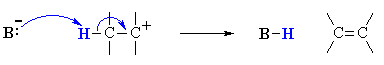
|
|
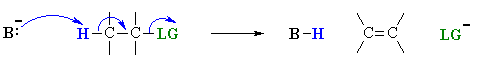 |
| © Dr. Ian Hunt, Department of Chemistry |