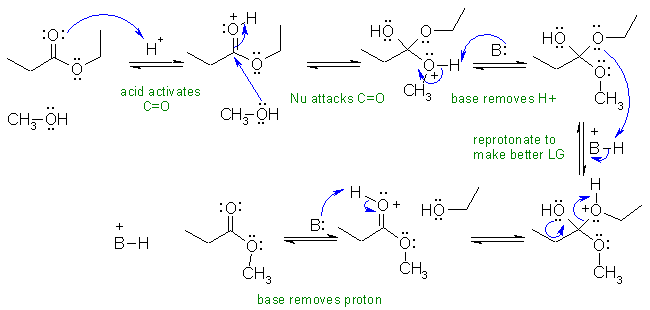
In this mechanism, B: could be ROH, the esters or -OR groups of the intermediate.
Note
that no other reagents are needed in order to complete any of these sequences,
you should only be using what is there.
Part A:
i. Transesterification : ethyl ester to methyl ester under acidic conditions.

In this mechanism, B: could be ROH, the esters or -OR groups of the intermediate.
Common errors: (1) The reaction conditions don't include water. (2) Since the reaction conditions are acidic, the carbonyl must be activated by the acid to promote attack by the Nu, the methanol. (3) Under acidic conditions, the leaving group is EtOH not ethoxide, EtO-. (4) If you use B: to represent the bases, then somewhere in the answer you should identify what B: is.
ii. Sulfonation of benzene, an electrophilic aromatic substitution.
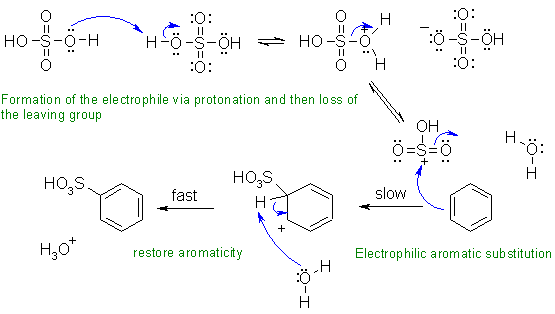
Common errors: (1) did not show the formation of the right electrophile (2) showed the H2SO4 reacting as a base to form a phenyl anion( yikes!) (3) did not show the C+ that is formed and the rearomatisation correctly.
iii. Reaction of a ketone with a primary amine derivative to give an oxime.
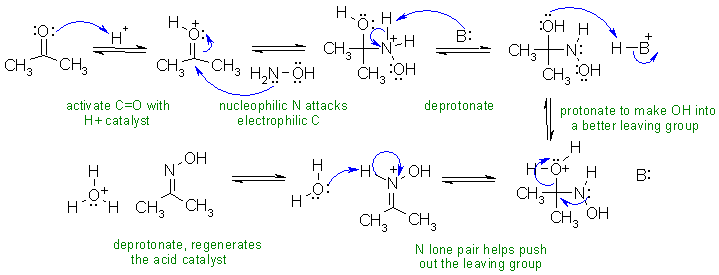
In this mechanism, B: could be C=O, OH or water
Common errors: (1) Not activating the carbonyl with the acid catalyst.
Part B:
i. The Prins reaction
Common errors: (1) Not activating the carbonyl with the acid catalyst. (2) Reacting the alkene to get an alcohol first (no route to the product this way) (3) not thinking about pKas and forming carbanions despite the acidic reaction conditions.
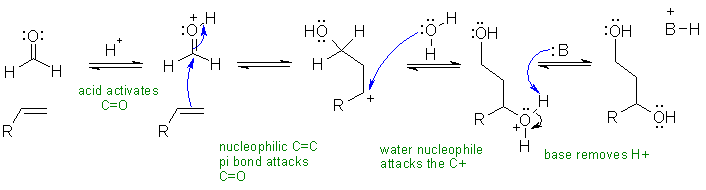
In this mechanism, B: could be the carbonyl group or the OH groups in water
or the alcohols.
ii An intramolecular aldol reaction then elimination of an alcohol to give a conjugated ketone (the enone product hints at the aldol mechanism).
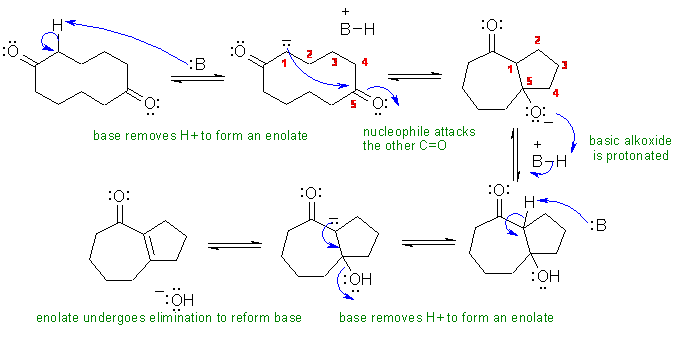
The base used in the question was defined as carbonate, CO32-.
The numbering is included here to help show the formation of the required ring system.
Common errors: (1) Getting the alkoxide in the wrong location after the attack of the enolate on the carbonyl. (2) forming H2O as the leaving group despite the basic reaction conditions. (3) Magic appearence of H atom on the formed -OH group.
Common general errors: (1) Ignoring reaction conditions (2) poorly drawn arrows, e.g. not starting at the Nu site (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not balancing charges (6) missing arrows esp. when adding or removing H+ (7) compressing several mechanistic steps into a single step.