Part 6: SYNTHESIS
Answers to the synthesis problems are given below.
These answers have been selected as they are short and efficient, but there
are probably other reasonable solutions. Note some targets have
specific stereochemistry that needs to be considered.
When planning
syntheses, one should try to work backwards by functional group analysis.
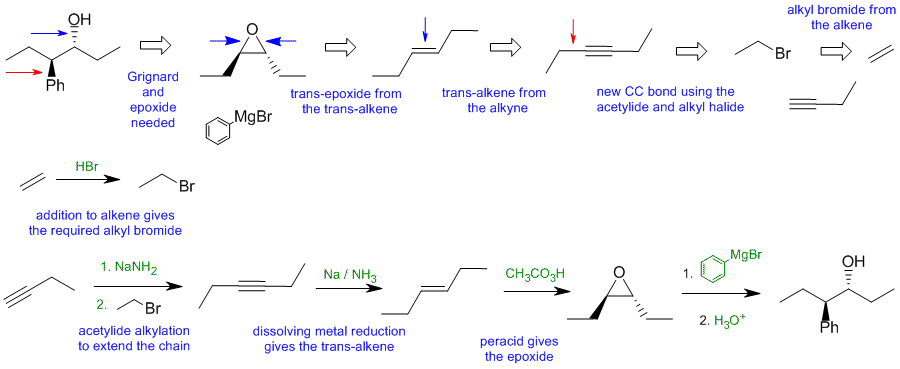
The diagrams show the "retrosynthesis" - the design or plan and then
below that the reaction scheme step-by-step (as required in the question). Red arrows try to show carbon-carbon bond forming reactions, blue arrows
are functional group manipulations. The blue text rationalise the retrosynthetic
analysis and information about important reactions are given in green.
Common errors:
- Lots of students made more use of Chem 351 reactions over 353 reactions. It's important to note that the 353 materials are often more selective and therefore give better control than the 351 reactions students elected to use.
- Not reading the instructions and using starting materials that were not hydrocarbons (C and H only) with 3 or less C or butadiene.
- Not counting carbons and therefore randomly adding or loosing C atoms and/ or changing the location of functional groups.
- Not controlling the location of functional groups (i.e. controlling regiochemistry)
- pKas ! i.e. not paying attention to the presence of acidic and basic groups.
- Reaction knowldege ? Need a LG to do SN or E reactions, wrong reagents for the desired task (e.g. alkyl halides eliminate with base not acid, alcohols with acid not base).
- Incorrect stereochemical consequences of addition reactions and SN reactions.
A1
![trans-2,3-dichlorobicyclo[2.2.2]octane](353mt17syna1.gif)
A2
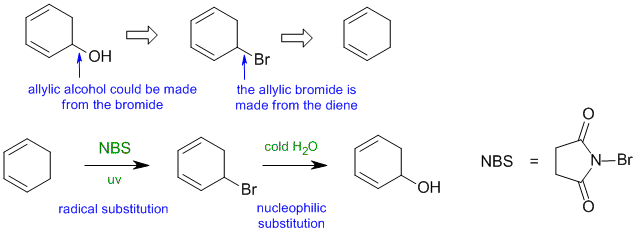
B1
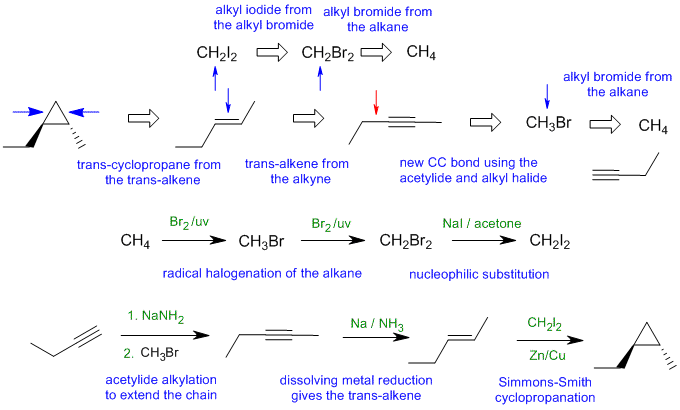
B2
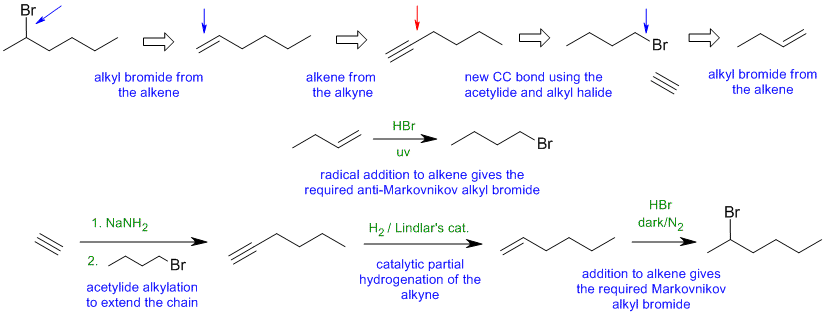
Common errors:
poor control of regioselectivity or wrong reagents.
Synthesis via hex-2-yne resulting in poor regioselectivity
C1
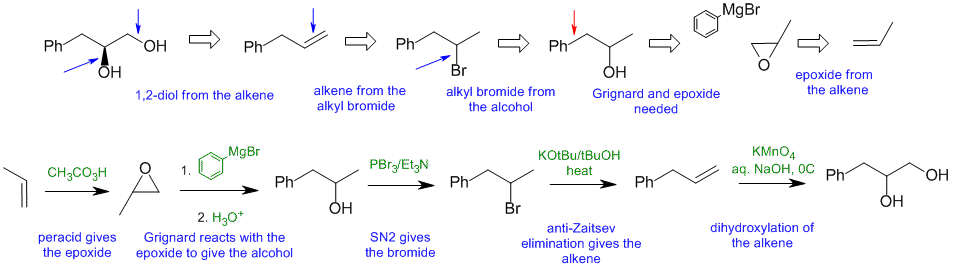
C2
![trans-2,3-dichlorobicyclo[2.2.2]octane](353mt17syna1.gif)
![trans-2,3-dichlorobicyclo[2.2.2]octane](353mt17syna1.gif)




