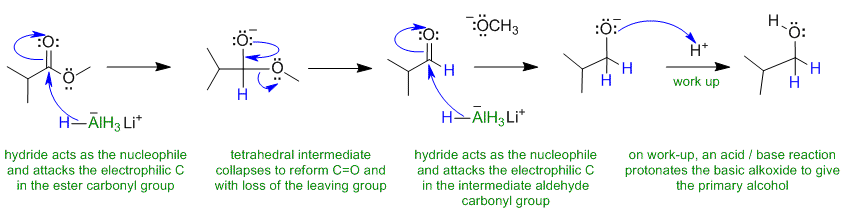
Note that no other reagents are needed in order to complete any of these sequences, you should only be using what is there.
A1Hydride reduction of an ester

A2 Acid catalysed amide hydrolysis
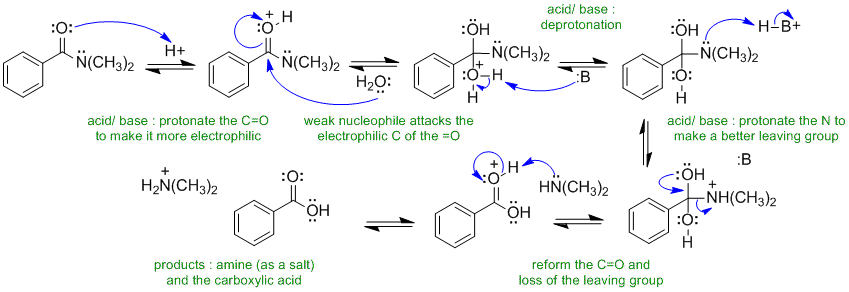
B: in this scheme could be the conjugate base of the acid catalyst, or water or one of the O or N atoms in the materials present.
B1 Blanc cyclisation :
Overview : The dicarboxylic acid starting material reacts with the anhydride to form a mix anhydride. The enol form of the ketone reacts with the mixed anhydride C=O followed by loss of carboxylate as a leaving group: As with many acid catalysed reactions, it looks complicated but the individual steps are not too bad....
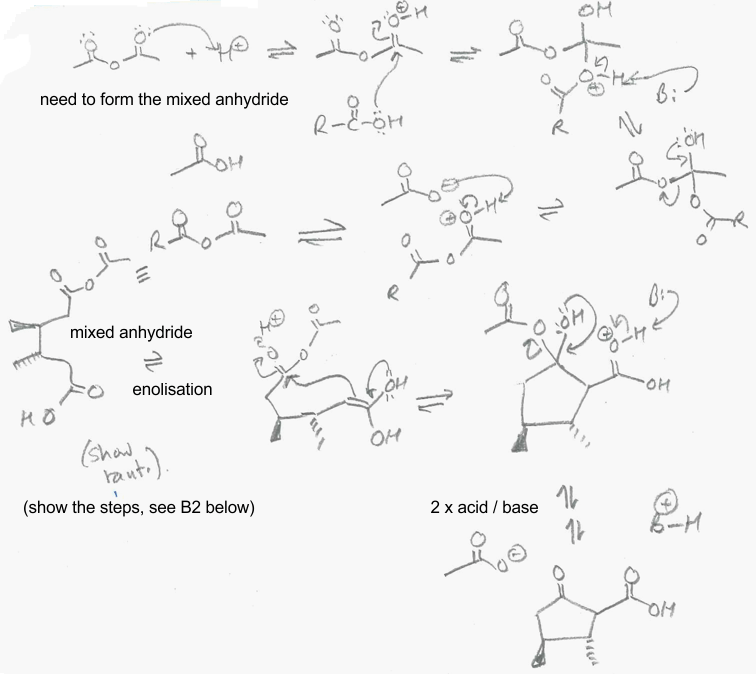
B2 Decarboxylation of a beta-keto acid derivative:
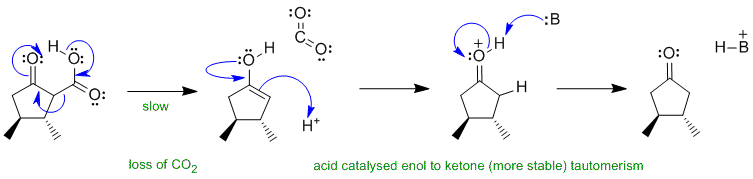
Note this this is the continuation of B1 above, so the reaction is under acidic conditions. The H+ could come from the carboxylic acid and the B: could be the carboxylate ion (conjugate base) or C=O systems present in the compounds.
Common general errors: (1) Ignoring reaction conditions i.e. effect of acidic or basic environment (2) poorly drawn arrows, e.g. vague (i.e. starting / ending "in space" (3) backwards arrows (4) wrong use of arrows e.g. resonance (5) not showing formal charges (6) missing arrows especially when adding or removing H+ (7) compressing several mechanistic steps into a single step (8) not knowing the basic mechanism types (9) drawing arrows that were inconsistent with the resulting bonding changes.