 |
|
 |
|
| Nomenclature |
Formula |
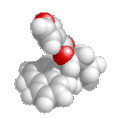
3D structure |
| Functional group suffix
= halide (i.e. fluoride, chloride, bromide, iodide) Substituent name = halo- (i.e. fluoro, chloro, bromo, iodo) Structural unit : haloalkanes contain R-X where X = F, Cl, Br, I etc. Notes :
|
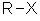 |
|
Haloalkane
style:
1-chloropropane
|
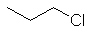 CH3CH2CH2Cl |
|
Alkyl
halide style:
tert-butyl bromide
Haloalkane style:
2-bromo-2-methylpropane
|
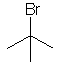 (CH3)3CBr
|
|
Haloalkane style:
4-bromobut-1-ene
|
CH2=CHCH2CH2Br |
| ©Dr. Ian Hunt, Department of Chemistry |