| Chapter 1: Structure Determines Properties |
| Chapter 1: Structure Determines Properties |
Representing 3D Molecular Shapes in 2D:
The JSMOL image below shows an interactive model of a molecule of methane, CH4 (you can use the mouse to turn the molecule). Figure I shows how this 3D image would be represented on a piece of paper. Unbroken lines are used to represent bonds in the plane of the paper, hashed wedges represent bonds going back out of the plane of paper and solid wedges represent bonds coming out of the plane of the paper. See if you can manipulate the molecule in the Chime image below to match the drawing in figure II.
CH4
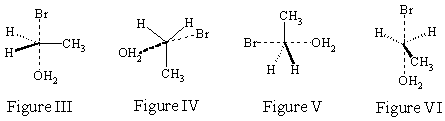
SN2 (later) reactions go via a trigonal bipyramidal transition state. The structures
below are from the reaction of ethyl bromide with water, CH3CH2Br + H2O. Can
you manipulate the trigonal bipyramidal transition state , given in the 3D JSMOL
image below, so that it matches the drawings in each of the figures immediately
to the right?
Notice that bonds that are in the process of being formed or broken are given as dashed lines. The representations that are clearest then are those that place those bonds in the plane of the paper so that they do not get confused with the dashed wedges that represent bonds coming out of the plane of the paper (Figure I III).
C2H7OBr
The most desirable drawings are usually the simplest, generally they place as many atoms as possible in the plane of the paper.
Now consider the molecule n-butane, JSMOL image given below.
Manipulate the molecule so that it matches the drawing in Figure IX.
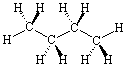
Figure IX |
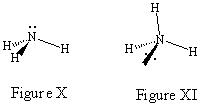
So what happens when lone pairs of electrons are involved? The most desirable drawings still place the most bonding sites in the plane of the paper even though lone pairs of electrons are most easily represented as being in the plane of the paper (it is not easy to wedge and hash a lone pair).
Consider the JSMOL image for NH3 given below. Figure
X would be the best drawing since it considers the lone pair on nitrogen as
being in a bonding site in the plane of the paper. As you can see in Figure
XI it is much more confusing if you choose to represent the lone pair as coming
out of the paper.
 |
Ethyl methyl ether is a structure where
the opposite is true.
Drawing the lone pairs in the plane of the paper would be far more
confusing
than if you consider them as being out of the plane.
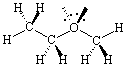 Figure XII |
You need to
learn to discriminate which
drawings most clearly represent the structure of a molecule.
 |
© Dr. Ian Hunt, Department of Chemistry, University of Calgary |  |