| Chapter 2 : Alkanes |
| Chapter 2 : Alkanes |
sp2 hybridisation
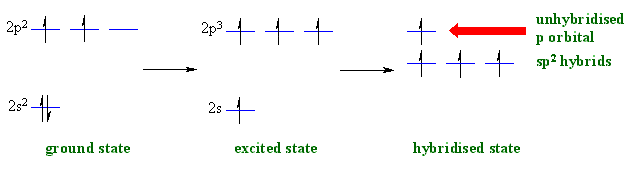
When a C atom is attached to 3 groups and so is involved
in 3 σ bonds, it requires 3 orbitals in the hybrid set. This requires that
it is sp2 hybridised. The general "steps" are similar to that
for seen previously sp3 hybridisation.
 |
|
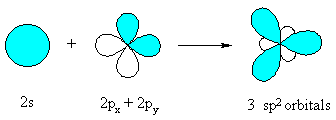
|
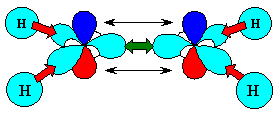
| You can view an animation of the hybridisation of the C orbitals if you wish. |
|
|
Summary
| © Dr. Ian Hunt, Department of Chemistry |