|
|
|
|
Conformational Analysis Questions
| Qu 1: |
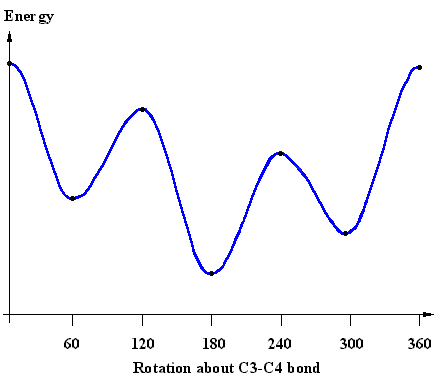
The energy diagram shows the relative energies of the conformations
of 2,2,3-trimethylpentane produced during the rotation about the C3-C4 bond.
Match the structural representations provided to the appropriate points on the energy profile. |
|
 |
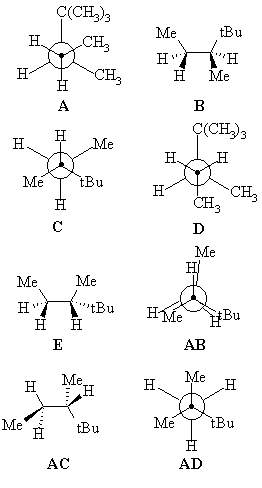 |
|
| Qu 2:
|
There are four possible stereoisomers of 1-tert-butyl-3-methylcyclohexanes
in which the cyclohexane is in a chair conformation. The calculated
heats of formation, ΔHf, of these four structures are listed
to the right.
Draw the four possible structures. Match the heats of formation, ΔHf, values to the appropriate structures Justify your choice. Using the available data, calculate : i) the heat of combustion, ΔHc, of the most stable structure ii) the equilibrium constant, K, for the interconversion of the two cis
forms at 25oC.
|
ΔHf (2) = -40.53 ΔHf (3) = -44.41 ΔHf (4) = -46.84 ΔHc (H2, gas) = -68.0 ΔHc (C, graphite) = -94.0 R = 1.987 cal/molK kJ/mol ΔHf(2) = -169.58 ΔHf (3) = -185.81 ΔHf (4) = -195.98 ΔHc (H2, gas) = -284.51 ΔHc (C, graphite) = -393.3 R = 8.314 J/molK |
| Qu 3: (tough)
|
Cyclopropane is unique among the cycloalkanes in that it can be hydrogenated
with H2 and Pt metal, giving propane. Similarly, propene
can be hydrogenated to give propane.
The heats of formation (ΔHf) for the hydrocarbons are: (1.) From this data, calculate the heats of hydrogenation (ΔHh) of cyclopropane and propene. (2.) Show this data on a typical energy diagram in which the enthalpies of propene + H2, cyclopropane + H2 and propane are given in rough relative positions. (3.) The bond energy of a C-C single bond is about 83 kcal/mol, a C=C double bond is about 146 kcal/mol. Using this data, together with the data from part (1), calculate a "ring strain" for cyclopropane, and compare this with the "ring-strain" value of 28 kcal/mol found experimentally from combustion data. |
 |
© Dr. Ian Hunt, Department of Chemistry |