| Chapter 4: Alcohols and Alkyl Halides |
| Chapter 4: Alcohols and Alkyl Halides |
SN1 mechanism
SN1 indicates a substitution, nucleophilic, unimolecular reaction, described by the kinetic expression : rate = k [R-LG]
This pathway is a multi-step mechanism with the following characteristics:
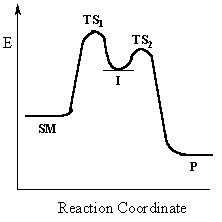 |
Multi-step reactions
have intermediates and several transition states
(TS).
In an SN1 reaction loss of the leaving group generates an intermediate carbocation which then undergoes a rapid reaction with the nucleophile. The reaction profiles shown here are simplified and do not include the equilibria for protonation of the -OH. |
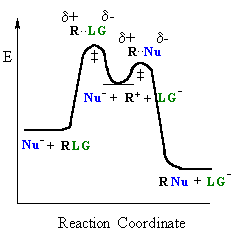 |
| General case | SN1 reaction |
The following issues are relevant to the SN1 reactions of alcohols, ROH :
The effect of the alkyl group: R-
Reactivity order : (CH3)3C-
> (CH3)2CH- > CH3CH2-
> CH3-
In an SN1 reaction, the key step is the loss of the leaving group to form the intermediate carbocation. The more stable the carbocation is, the easier it is to form, and the faster the SN1 reaction will be. Some students fall into the trap of thinking that the system with the less stable carbocation will react fastest, but they are forgetting that it is the generation of the carbocation that is rate determining.
The effect of the leaving group: -LG
The only event in the rate determining step of the SN1 is
breaking
the C-LG bond. For alcohols
it is important to remember that -OH is a very poor leaving. In the
reactions
with HX, the -OH is protonated first to give an oxonium, providing the
much better leaving group, a water molecule (see scheme below).
The effect of the nucleophile: Nu
Since the nucleophile is not involved in the rate determining step
of an SN1 reaction (since rate = k [R-LG]), the nature of the nucleophile is
unimportant.
In the reactions of alcohols, ROH, with HX, the reactivity trend of HI >
HBr
> HCl > HF is not due to the nucleophilicity of the halide ion
but the
acidity of HX which is involved in generating the leaving group prior
to
the rate determining step.
Stereochemistry
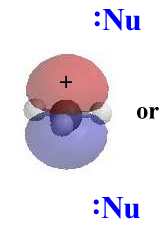 |
In an SN1 reaction, the nucleophile attacks
the planar carbocation. Since there is an equally probability of attack
on either face there will be a loss of stereochemistry at the reactive
center and both possible products will be observed.
|
Since a carbocation intermediate is
formed, there is the possibility
of rearrangements (e.g. 1,2-hydride or 1,2-alkyl shifts) to
generate
a more stable carbocation (see later). This is usually indicated
by a change in the position of the halide compared to that of the
original
-OH group, or a change in the carbon skeleton of the product when
compared
to the starting material.
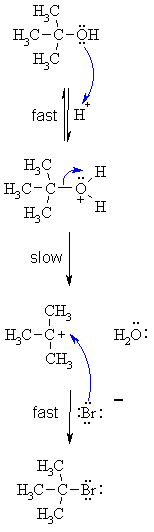
SN1
MECHANISM FOR REACTION OF ALCOHOLS WITH HBr |
|
Step
1: Step 2: Step 3:
|
 |
| © Dr. Ian Hunt, Department of Chemistry |