|
Structure and Preparation of Alkenes. Elimination Reactions |
|
Structure and Preparation of Alkenes. Elimination Reactions |
E1 mechanism
E1 indicates a elimination, unimolecular
reaction, where rate = k [R-LG].
This implies that the rate determining step of the mechanism depends on the
decomposition of a single molecular species.
Overall, this pathway is a multi-step process with the following
two critical steps:
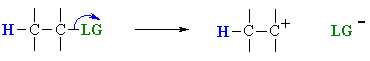 |
loss of the leaving group, LG, to generate a carbocation intermediate, then | |
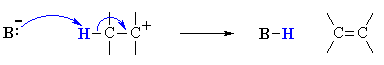 |
loss of a proton, H+, from the carbocation to form the π-bond |
Let's look at how the various components of the reaction influence the reaction pathway:
R-
Reactivity order : (CH3)3C- >
(CH3)2CH- > CH3CH2-
> CH3-
In an E1 reaction, the rate determining step is the loss
of the leaving group to form the intermediate carbocation. The more
stable the carbocation is, the easier it is to form, and the faster the E1 reaction
will be. Some students fall into the trap of
thinking that the system with the less stable carbocation will react fastest,
but they are forgetting that it is the generation of the
carbocation that is rate determining.
Since carbocation intermediates are formed during an E1, there is always
the possibility of rearrangements (e.g. 1,2-hydride or 1,2-alkyl shifts)
to generate a more stable carbocation. This is usually indicated by a change
in the position of the alkene or a change in the carbon skeleton of the product
when compared to the starting material.
-LG
The only event in the rate determining step of the E1 is breaking the C-LG
bond. Therefore, there is a very strong
dependence on the nature of the leaving group, the better the leaving group,
the faster the E1 reaction will be. In the acid catalysed reactions
of alcohols, the -OH is protonated first to give an oxonium ion, providing the
much
better leaving group, a water molecule (see scheme below).
B
Since the base is not involved in the rate determining step, the nature of the
base is unimportant in an E1 reaction. However, the more reactive the base,
the more likely an E2 reaction becomes.
Selectivity
E1 reactions usually favour the more stable alkene as the major product : i.e. more
highly alkyl substituted and trans- > cis-
This E1 mechanistic pathway is most common with:
|
|
|
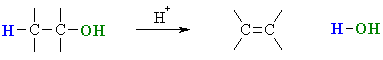 |
|
| Step 1: An acid/base reaction. Protonation of the alcoholic oxygen to make a better leaving group. This step is very fast and reversible. The lone pairs on the oxygen make it a Lewis base. |
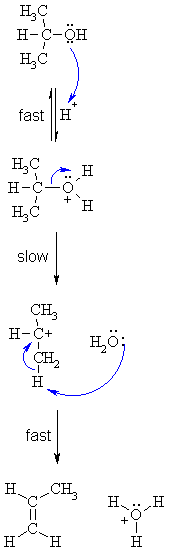 |
| Step 2: Cleavage of the C-O bond allows the loss of the good leaving group, a neutral water molecule, to give a carbocation intermediate. This is the rate determining step (bond breaking is endothermic) |
|
| Step 3: An acid/base reaction. Deprotonation by a base (a water molecule) from a C atom adjacent to the carbocation center leads to the creation of the C=C |
|
|
|
|
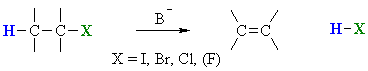 |
|
| Step 1: Cleavage of the polarised C-X bond allows the loss of the good leaving group, a halide ion, to give a carbocation intermediate. This is the rate determining step (bond breaking is endothermic) |
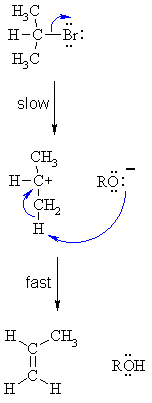 |
| Step 2: An acid/base reaction. Deprotonation by a base (here an alkoxide ion) from a C atom adjacent to the carbocation center leads to the creation of the C=C |
|
| © Dr. Ian Hunt, Department of Chemistry |