|
Structure and Preparation of Alkenes. Elimination Reactions |
|
Structure and Preparation of Alkenes. Elimination Reactions |
In many cases elimination reactions may proceed to give alkenes that are isomeric but with one formed in excess of the other (i.e. a major product).
Regioselectivity (products are constitutional isomers):
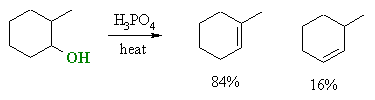
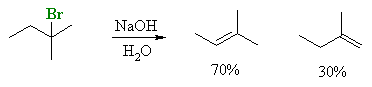
Zaitsev's rule, based on experiment observations
of the dehydration of alcohols, expresses the preference for eliminations
to give the highly substituted (more stable) alkene, which may also be
described as the Zaitsev product.
The rule is not always obeyed, some reactions give the anti-Zaitsev product
which is sometimes described as the Hoffman product. (Hoffman studied the elimination of ammonium salts).
Care is needed with E2 eliminations of cyclic systems since the antiperiplanar
alignment of the C-H and C-LG bonds
can dictate that the anti-Zaitsev products dominate.
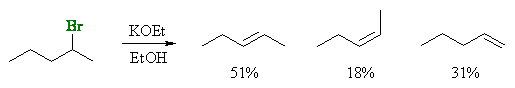
Stereoselectivity (products are stereoisomers)
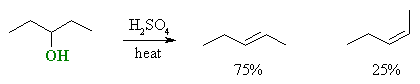
| © Dr. Ian Hunt, Department of Chemistry |